Return to Chapter 45 Chemical Hygiene and Safety Plan.
DRAFT
Contents
Approved by Evelyn Davies
Revised 5/2025
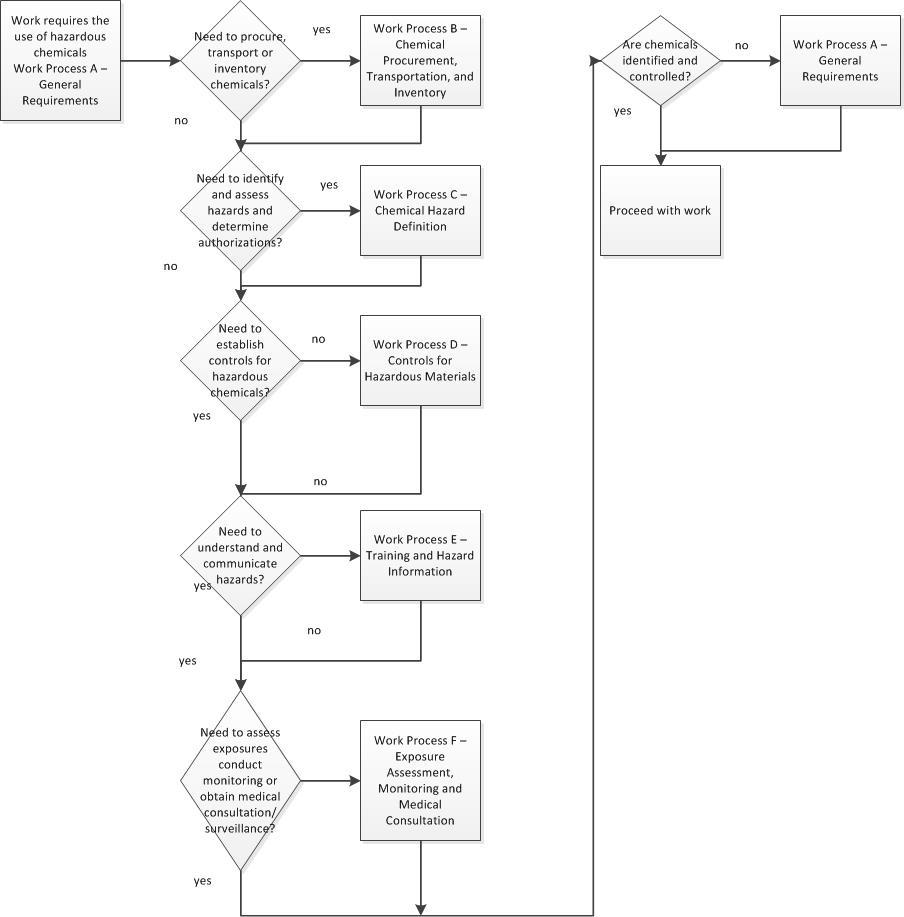
Work Process A. General Requirements
Work Process B. Chemical and Equipment Procurement
Work Process C. Transporting Hazardous Materials
Work Process D. Berkeley Lab Chemical Inventory
Work Process E. Chemical Hazard Evaluation: Identification, Classification, and Categorization
Work Process F. Use and Application of Chemical Hazard Assessments
Work Process G. General Controls for Hazardous Chemicals
Work Process H. Selection and Use of Engineering Controls
Work Process I. Personal Protective Equipment
Work Process J. Work Practice Controls
Work Process K. Chemical Storage
Work Process L. Specific Controls and Procedures — Acids and Bases
Work Process M. Specific Controls and Procedures — Particularly Hazardous Substances: Carcinogens, Reproductive Toxins, and Acute Toxins
Work Process N. Specific Controls and Procedures — Flammables and Combustible Liquids
Work Process O. Specific Controls and Procedures — Laser Dyes and Solvents
Work Process P. Specific Controls and Procedures — Peroxide-Forming Compounds
Work Process P.1 Specific Controls and Procedures — Additional Time-Sensitive Chemicals
Work Process Q. Specific Controls and Procedures — Water-Reactive Chemicals
Work Process R. Specific Controls and Procedures — Pyrophoric Materials
Work Process R.1 Specific Controls and Procedures — Chemical Synthesis
Work Process S. Specific Controls and Procedures — Engineered Nanomaterials
Work Process T. Specific Controls and Procedures — Chemicals with Explosive Properties
Work Process U. Decommissioning or Transferring Equipment, Buildings, Laboratories, and Shop Spaces
Work Process V. Emergency Procedures and Equipment
Work Process W. Training
Work Process X. Safety Data Sheets
Work Process Y. Container Labeling
Work Process Z. Hazard Communication Requirements for Chemicals Produced in LBNL Laboratories
Work Process AA. Posting Area Entrances
Work Process BB. Designated Areas
Work Process CC. Exposure Assessments, Monitoring, and Medical Consultation
Work Process A. General Requirements
- Chemical Safety Work Process Flowchart
- Employee Rights. All personnel have the right to:
- Be notified of measured or suspected exposures to harmful substances at or above occupational exposure limits
- Request medical consultations and access their workplace medical and exposure records, and for their physician or collective bargaining agent to receive information regarding hazardous chemicals to which the employee may be exposed
- Refuse to work in unsafe conditions or to perform work that could create a hazard to themselves or other workers
- Protection against discharge or other discrimination due to exercising the rights afforded pursuant to the provisions of the Hazardous Substances Information and Training Act
- File confidential health and safety complaints with the UC Whistle blower Hotline at (800) 403-4744
Work Process B. Chemical and Equipment Procurement
- General Information. LBNL expects individuals to understand the risks of hazardous chemicals they purchase or allow onsite and to minimize the risks of those chemicals through elimination, substitution, reduction, use of engineering/administrative controls, and use of personal protective equipment where necessary. Pre-Procurement Assessment Requirements for Hazardous Chemicals in Section 2 must be met for all chemicals to be allowed onsite. All hazardous materials require screening and approval prior to arrival on-site. Screening and approval requirements for hazardous chemicals are determined by the acquisition route outlined in Section 3.
In addition, some chemicals and equipment have inherent safety hazards that require special safety controls and authorizations. It is important that these controls are in place before the material is procured and used on site. Materials of concern are listed in the Restricted Items List that is maintained by the Procurement Department in consultation with EHS (Section 4). None of the items on this list may be purchased using credit cards such as Division PCards or corporate credit cards. Section 4 also includes the list of chemical hazard categories that are considered “high hazard chemicals” per Work Planning and Control (high risk, Risk Level 3), and require an approved Work Activity prior to acquisition.
- Pre-procurement Assessment Requirements for Hazardous Chemicals
- LBNL expects individuals making the decision to allow a hazardous chemical onsite to do the following:
- Plan the work that requires the hazardous chemical (or notify the Activity Lead responsible for planning the work of hazardous chemicals that will be used)
- Have a plan for the disposal of hazardous waste
- Prepare for the safe use and storage of the hazardous chemical
- Ensure facilities are adequate, necessary safety controls are implemented, and individuals using chemicals are qualified
- Know if hazardous chemicals are on the restricted items list and obtain institutional approval for the use of those chemicals including chemicals brought onsite by other institutions (Section 3)
- Borrow what you need when possible
- Use less hazardous chemicals when possible
- Purchase only what is needed for the short term or project; plan for minimum waste and leftover unused hazardous chemicals
- Start using last of existing stock before buying new supplies (do not stockpile)
- The SDS should be reviewed prior to using hazardous chemicals. Refer to Work Process E for assessing chemical hazards. An EHS Health and Safety Representative may also be consulted for guidance.
- Some equipment contains chemicals that can pose a hazard when mishandled, such as elemental mercury in porosimetry (instruments for characterizing a material’s porosity by applying various levels of pressure to a sample immersed in mercury). When normal operation or reasonably foreseeable mishandling of chemical-containing equipment may result in a loss of control (such as a spill) that could pose an exposure hazard, cause extensive area contamination, or result in environmental damage, the Activity Lead must assess the hazards and implement controls. An EHS Division subject matter expert may be consulted. Moreover the Work Planning and Control (WPC) Activity for the work using the equipment shall address its off-normal consequences/hazards and the associated controls.
- LBNL expects individuals making the decision to allow a hazardous chemical onsite to do the following:
- Screening and Approval Requirements (Chemical Acquisition Resources)
- The acquisition of hazardous chemicals (including all compressed and liquefied gas cylinders) is subject to a hazard-based screening and approval process prior to arrival on site. Chemicals that meet the screening criteria and require further approval from EHS and/or Security and Emergency Services (including the Fire Protection Group) will be routed to the appropriate parties for approval based on the hazard, quantity, and location.
- For chemicals ordered through LBNL’s Financial Management System (FMS), including eBuy and ePro, the hazardous chemical procurement decision maker (e.g., Requestor) must indicate whether each requisition line item being ordered is a hazardous chemical or compressed or liquified gas by answering YES then entering the required information. Answering YES will automatically create an inventory record in CMS and information will be sent for screening and approval in CMS prior to the order proceeding.
- For hazardous chemicals (including compressed or liquified gas cylinders) that require CMS tracking to be acquired outside of FMS, including, but not limited to, primary manufacturer containers from collaborators or those acquired through a non-LBNL purchasing system and transported to LBNL, the Requestor must ensure these chemicals are added directly in CMS and approved prior to arrival on site (i.e. prior to ordering). Chemicals must be added in CMS with “ordered” status and will be routed for the same screening and approval process as for chemicals ordered in FMS.
- If a chemical to be acquired does not require CMS tracking (e.g. research samples), the Requestor is responsible for assuring the scope of work, including hazards, controls, and material quantities are covered by an authorized Work Activity.
- In any case, the Requestor is accountable for obtaining necessary approvals prior to arrival onsite, including approvals for materials falling under the Physical Security Program’s hazardous materials management policy and the Procurement Restricted Items List (see Section 4 below). Approval is facilitated by EHS and will be contingent upon verification that a Work Planning and Control Activity authorizing the scope of work is current and adequate facilities and controls are in place.
- The acquisition of hazardous chemicals (including all compressed and liquefied gas cylinders) is subject to a hazard-based screening and approval process prior to arrival on site. Chemicals that meet the screening criteria and require further approval from EHS and/or Security and Emergency Services (including the Fire Protection Group) will be routed to the appropriate parties for approval based on the hazard, quantity, and location.
- Restricted Items List and High Risk Chemical Hazard Categories
- The Restricted Items List includes chemicals and equipment, such as:
- Biological agent
- Biosafety cabinets
- Chemicals and gases (CHEMR):
- lethal/incapacitating toxicants and relevant precursors
- hazardous and reactive gases
- explosive and potentially explosive materials
- alkali metals
- select water-reactive chemicals
- pyrophoric liquids and solids (GHS)
- beryllium and beryllium-containing compound
- unstable and reactive materials
- Cal/OSHA regulated carcinogens
- peroxide-forming chemicals: Group A (may form explosive peroxides without concentration – test every 3 months)
- refrigerants and ozone-depleting substances (sulfur hexafluorixde – SF6)
- Chemical storage cabinets
- Fire extinguishers
- Fume hoods
- Gas storage cabinets
- Hazardous and toxic gases
- Laminar airflow hoods
- Laser equipment (excluding laser pointers)
- Radioactive isotopes
- Refrigerators and freezers for flammable liquid storage
- Respiratory protection equipment
- X-ray equipment
- Refer to the Restricted Items List for the Point of Contact (POC) for specific materials and equipment.
- High Risk Chemical Hazard Categories per Work Planning and Control (i.e. high hazard chemicals, Risk Level 3) have the highest degree of hazard, in which a loss of control could result in immediate injury or death, and therefore require a higher level of control and authorization. Risk Level 3 Chemical Hazards include:
- Corrosive, pyrophoric and toxic gases (See also Chapter 13)
- Explosives (primary and secondary)
- High acute toxicity chemicals (GHS Acute Toxicity Category 1 and NFPA Health Hazard Rating 4)
- Hydrofluoric acid and hydrofluoric acid-generating/releasing materials (such as hydrogen fluoride pyridine and buffered oxide etchant)
- Pyrophoric liquids and solids
- Tetramethylammonium hydroxide (TMAH) and solutions, etchants, and developers containing greater than 1% TMAH
- Water reactives (GHS substances or mixtures which, in contact with water, emit flammable gases, category 1, including alkali metals)
- See Work Process F. for examples of specific materials of concern.
- Refer to Work Planning and Control Activity Manager for applicable controls and additional information.
- Both CHEMR and high hazard chemicals (on a hazard-screening basis) require approval to verify that pre-acquisition requirements in Section 2 were met. As such, the Requestor should include the applicable WPC Activity ID during acquisition (Section 3) as required information to avoid delays.
- The Restricted Items List includes chemicals and equipment, such as:
Work Process C. Transporting Hazardous Materials
- Transporters
- Hazardous materials will be transported by Transportation Services or a Department of Transportation (DOT) authorized carrier (except as outlined below). Transporting hazardous materials via public transportation (such as the shuttle bus) or in private or government vehicles is not permitted. This is to minimize risk to Laboratory employees and the public. This also ensures that the federal and state laws regarding packaging, manifesting, and placarding hazardous materials are met. There are exceptions for transporting research samples, hazardous materials, and field sampling materials, described later.
- The following parties are permitted to transport hazardous materials between non-adjacent Berkeley Lab buildings and from off-site locations (e.g., UC Berkeley campus):
- Transportation Services (ext. 5404) will transport hazardous materials, provided they are unopened and still in their original DOT shipping containers. They will also transport gas cylinders. In addition, Berkeley Lab Transportation Services will package and label hazardous materials in accordance with DOT Title 49 regulations for shipment by commercial carriers.
- SDSs and hazard warning container labels are required for off-site shipments of chemicals that are produced in LBNL laboratories. Refer to Work Process Z for hazard communication requirements for chemicals produced in laboratories.
- The EHS Waste Management Group (ext. 5877) will transport hazardous materials that have previously been opened. This is normally needed for laboratory moves.
- Facilities craft personnel will transport paints, solvents, cleaners, and other materials necessary to perform their work.
- Transporting Small Quantities of Hazardous Materials by Laboratory Employees, Subcontractors, and Affiliates
- Laboratory employees, subcontractors, and affiliates may move small quantities of hazardous materials for short distances within a building or between adjacent buildings, provided it can be done safely and without spilling the materials. Individuals must use a means of containing leaks and spills such as handcarts and drip trays, or bottle carriers. Employees must also complete Chemical Hygiene and Safety Training (EHS0348; or 345 for Facilities personnel). Use standard cylinder dollies to transport compressed gas cylinders. While dollies are preferred, cylinders weighing 11 kg (25 lbs) or less may be hand-carried.
- Never move a cylinder with a regulator connected to it. Cylinder valve-protection caps and valve-opening caps must be in place when moving cylinders. Lecture bottles and other cylinders not normally equipped with valve-protection caps should be transported in either the original DOT specification package or an equivalent container.
NOTE: Contact an EHS Radiological Control Technician for guidance on transporting radiological isotopes.
- Transporting Research Samples, Hazardous Materials, and Field Sampling Materials by Berkeley Lab Staff
- Policy and Procedures
- The policy for transporting research samples, hazardous materials, and field sampling materials by Berkeley Lab staff is established in the ES&H Manual Chapter 54 Transporting and Shipping Hazardous Materials program. The process described below is the procedure for implementing this policy.
- Berkeley Lab staff (i.e., anyone with a Berkeley Lab badge) may transport research samples and small quantities of hazardous materials by hand or in a passenger vehicle under the conditions defined by procedures described below. “Small quantities” is defined in Section 4, Scope and Application below. A research sample is a material used or developed in a laboratory for research purposes, for further use, study, analysis, or characterization.
- Shipping samples and hazardous materials by common carrier (FedEx, UPS, USPS) to off-site locations must be done by Facilities Material Services. This is addressed in Pub 3000 Chapter 54 Transporting and Shipping Hazardous Materials Work Process E, Shipping.
- Questions regarding this policy may be addressed to the EHS Deputy Division Director.
- Policy and Procedures
- Scope and Application
- This policy applies to Berkeley Lab staff who:
- Transport research samples (including engineered nanomaterials) and hazardous chemicals between:
- Buildings at the main Berkeley Lab site
- The main Berkeley Lab site and other Laboratory sites (e.g., Donner, Potter, JBEI, JGI)
- Any Berkeley Lab site and other collaborating research organization (e.g., UC Berkeley and Stanford)
- Transporting small quantities of hazardous materials to and from field locations not readily served by common carriers such as FedEx and UPS.
- Transport research samples (including engineered nanomaterials) and hazardous chemicals between:
- This policy applies to research samples and hazardous materials in the following DOT hazard categories. These materials must be contained in proper packaging (see below) and shall not exceed 0.5 kg (1 lb) or 0.5 L (1 pint) gross packaging size. To determine hazard class, consult a Safety Data Sheet (SDS) or contact the EHS Transportation Subject Matter Expert.
- Class 3 (flammable liquid)
- Class 8 (corrosive material)
- Class 9 (other regulated material)
- Division 4.1 (flammable solid)
- Division 5.1 (oxidizer)
- Division 5.2 (organic peroxide)
- Division 6.1 (toxic or poisonous)
- ORM-D (other regulated material, consumer commodity)
- Contact the EHS Deputy Division Director for exemptions to the above hazard class/quantity limitations, or for transporting materials in the following DOT hazard categories:
- Division 2.1 (flammable gas)
- Division 2.2 (nonflammable gas)
- This policy applies to Berkeley Lab staff who:
- Prohibitions. This policy does not apply to biological materials and materials that are radioactive, self-reactive, pyrophoric, explosive, water-reactive, acutely toxic by inhalation, or hazardous waste. “Acutely toxic” refers to substances that may be fatal or cause damage to target organs from a single exposure or from exposures of short duration. It also includes materials capable of causing intense irritation that can result in pulmonary edema (fluid and swelling in the lungs), chemical asphyxia, and systemic (body-wide) poisoning. A substance’s acute toxicity may be determined by consulting an SDS. Contact the EHS Transportation Subject Matter Expert for further information.
- Personnel Qualifications
- Staff transporting hazardous materials must have Chemical Hygiene and Safety Training (EHS0348), Chemical Hygiene and Safety Refresher Training (EHS0353) as applicable, and Safe Handling of Engineered Nanoscale Particulate Matter (EHS 0344) if they transport engineered nanoscale particulate matter. Furthermore, transporting research samples and small quantities of hazardous materials must be authorized in an employee’s WPC Activity.
- Facilities Material Services personnel who package materials for common carrier shipment must be qualified in accordance with the DOT or the International Air Transport Association (IATA) regulations.
- Packaging Requirements for Hand Carrying and Transportation by Vehicle
- An inner receptacle and outer packaging are required (see photos below):
- Containers can be procured from a variety of companies such as Grainger (formerly called Lab Safety Supply) or HAZMATPAC, Inc.
- The inner receptacle must be:
- Leak-tight, securely closed, and protected against damage. A screw-type cap or other positive means of closure is required. Parafilm, aluminum foil, and stoppers are prohibited.
- Labeled with the identity of the material, its hazard, the name and phone number of the sender, and the name and phone number of the recipient (if different from the sender). The chemical identity must be the common name or the DOT proper shipping name. Chemical formulas, abbreviations, or acronyms are prohibited. If the material is an engineered nanomaterial, include the following words on the label:
“Nanoscale — This material’s toxicity, reactivity, and other hazards may be greater than its macro-size forms” - Placed in a zip-lock bag or equivalent to serve as secondary containment in the event of a leak
- Sealed in an outer package
- The outer packaging must:
- Be made of rigid material such as a cardboard or plastic, or a metal box or pail
- Contain cushioning material to prevent breakage and to maintain each inner receptacle in an upright condition
- Have the same labeling as the inner receptacle
- Note: multiple chemicals in the same outer package must be chemically compatible with each other
| Inner receptacle with positive closure and label | Zip-lock bag to contain leaks/spills | Outer packaging |
- Communication of Chemical Hazards in Transport
- Staff transporting material must inform vehicle passengers about the research samples/hazardous materials being transported and the requirements of this policy.
- An SDS must be placed inside the outer package.
- Other Requirements for Staff Hand-carrying and Transporting by Vehicle
- Maintain possession and control of the material at all times.
- Transport the material directly to its final destination with no intermediate stops.
- Using Berkeley Lab shuttle buses and other modes of public transit is prohibited.
- Keep hazardous materials in the car trunk or truck bed. Do not transport in the passenger compartment.
- Use a DOT- or OSHA-approved safety can with a rated volume of 5 gallons or less to transport gasoline to field locations. Keep it in the open bed of a truck. Do not transport in the trunk or compartment of a passenger vehicle.
- DOT placarding is not required for vehicles provided that the quantity and classification criteria described in Scope and Application (above) are followed.
- Keep purchased materials intended for field use in the manufacturer’s original packaging. Otherwise, follow the packaging requirements as described above.
- Update the Chemical Management System for applicable containers moved to another location.
- Requirements for Shipping by Common Carrier
- The Cal/OSHA Hazard Communication Standard (8 CCR 5194) requires SDSs and container labels for research samples and hazardous chemicals that are shipped from laboratories. There is no exemption based on size or volume. Refer to Work Process Z for additional details and hazard communication requirements.
- ES&H Manual Chapter 54, Transporting and Shipping Hazardous Materials Work Process E, Shipping, also requires that only qualified individuals in Facilities Material Services (ext. 5084) may pack and ship these materials off site. Berkeley Lab employees intending to ship research samples and hazardous materials off site must:
- Label the material as described in Work Process C, Hand-Carrying or Self-Transport by Hand and Foot; and Work Process D, Self-Transport by Vehicle, of the Transporting and Shipping Hazardous Materials program. Also list the sender’s address.
- Prepare an SDS to be shipped with the material. Contact the Chemical Hygiene and Safety (CHSP) manager for guidance.
- Coordinate pickup with Facilities Material Services (ext. 5084).
- Notify the recipient prior to transport.
- Facilities Material Services shall:
- Arrange for pickup of the material
- Pack the material in accordance with DOT and/or IATA regulations
Work Process D. Berkeley Lab Chemical Inventory
- Purpose
- The purpose of the site-wide chemical inventory is to provide chemical users, EHS staff, and emergency response teams with accurate and up-to-date lists of hazardous chemicals stored on site. Furthermore, Cal/OSHA requires that a hazardous chemical list be maintained. Current chemical inventory reports must also be provided for compliance with DOE and City of Berkeley regulations. The inventory is also used to categorize chemicals into their respective hazard classes and to use this information as a tool to:
- Identify users of particular materials
- Communicate hazard information, including special controls or procedures
- Assist EHS in helping chemical users to determine if exposure assessments are needed
- Use of the chemical inventory permits emergency personnel to respond to unplanned releases with appropriate training, equipment, and organizational support. A well-maintained chemical inventory can also aid in internal Laboratory management of business and research needs.
- The purpose of the site-wide chemical inventory is to provide chemical users, EHS staff, and emergency response teams with accurate and up-to-date lists of hazardous chemicals stored on site. Furthermore, Cal/OSHA requires that a hazardous chemical list be maintained. Current chemical inventory reports must also be provided for compliance with DOE and City of Berkeley regulations. The inventory is also used to categorize chemicals into their respective hazard classes and to use this information as a tool to:
- Responsibilities
- The EHS Division hosts the site-wide chemical inventory database within the Chemical Management System (CMS).
- All hazardous materials are required to be included in the CMS. SDSs for materials should be consulted to determine hazard status. An EHS Health and Safety Representative may be consulted to help determine the hazards of a chemical or product.
- Division Directors are responsible for ensuring a full, wall-to-wall chemical inventory reconciliation is completed within their division at least once every two years, which will include laboratory and technical area safety surveys performed by EHS. They are also responsible for the timely resolution of non compliances resulting from these activities.
- Chemical Owners
- The chemical owner must maintain an accurate inventory of all hazardous chemicals/materials for which they are responsible for in the Chemical Management System (CMS). This includes ensuring hazardous chemicals and gases are inventoried in CMS with the correct information and that the container records are disposed of in the inventory when the materials have been used up, disposed of, or taken off-site.
- The chemical owner is accountable and responsible for the proper labeling, testing, management, and recordkeeping for his/her peroxide-forming and other time sensitive chemicals. The chemical owner may choose to assign time sensitive chemical labeling, testing, management and recordkeeping tasks to his/her proxies.
- Before vacating an area or leaving the Lab, the chemical owner must make sure that all hazardous materials are removed, transferred to new ownership, or properly disposed of.
- A chemical owner is accountable for the safe storage of hazardous chemicals, though all chemical users share the responsibility to use and store hazardous materials safely. Safe chemical usage is a Line Management responsibility.
- A chemical owner must provide the resources to make safe storage possible. This includes the purchase of equipment and accessories — such as cabinets and storage containers for flammables and corrosives — to control hazards. The chemical owner must have the authority to set administrative controls such as procedures for safe storage of chemicals. Guests, students, visiting scientists, and other short-term staff usually do not meet these requirements, but it is up to each division to assign ownership of chemicals and hazardous materials.
- Chemical Management System (CMS)
- There are two ways chemicals are added to CMS, depending on their method of acquisition (Chemical Acquisition Resource).
- For chemicals ordered through LBNL’s Financial Management System (FMS), including eBuy and ePro, purchase requesters must indicate whether each requisition line item being ordered is a hazardous chemical or compressed or liquified gas by answering YES then entering the required information. Answering YES will automatically create an inventory record in CMS and information will be sent for screening and approval prior to the order proceeding.
- For hazardous chemicals (including compressed or liquified gas cylinders) that require CMS tracking to be acquired outside of FMS, including, but not limited to, primary manufacturer containers from collaborators or those acquired through a non-LBNL purchasing system then transported to LBNL, the hazardous chemical procurement decision maker (e.g., Requestor) must ensure these chemicals are added directly in CMS prior to arrival on site. Chemicals must be added in CMS with “ordered” status and will be routed for the same screening and approval process as for chemicals ordered in FMS.
- If a container does not require CMS tracking (e.g. research samples), the Requestor is responsible for assuring the scope of work, including hazards, controls, and material quantities are covered by an authorized Work Activity.
- In any case, necessary approvals must be in place prior to arrival onsite, including approvals for materials falling under the Physical Security Program’s hazardous materials management policy and the Procurement Restricted Items List.
- The CMS identifies containers (or groups of identical containers) with a radio-frequency identification (RFID) Tag containing a seven-digit ID number. The tag should be affixed to the container with the ID number completely visible. Refer to the RFID Tagging Guide for resources on applying RFID tags on containers. RFID tags can be obtained through chemical management CMS@lbl.gov or via request form.
- The following information must be in CMS for each chemical:
- Container RFID Tag ID number
- Chemical or product name
- Chemical or product concentration
- Container size
- Container unit (kg, l, ml, etc.)
- Container type (glass bottle, can, etc.)
- Physical state (solid, liquid, gas)
- Manufacturer
- Temperature
- Pressure
- Building
- Room
- Owner
- Note: When entering groups of materials or materials with a high throughput rate in a specific room, such as commonly used organic solvents, acids, and bases, a CMS record can represent multiple identical containers in CMS, with exceptions noted under 5.g. This is referred to as a static RFID tag or a multi-container record. In such cases, the maximum amount possible should be indicated in the Size/Unit of the record.
- Contact Chemical Management System Support at CMS@lbl.gov for information on inventory implementation and training.
- There are two ways chemicals are added to CMS, depending on their method of acquisition (Chemical Acquisition Resource).
- Content Guidance (Content Guidance Resource)
- All hazardous materials/chemicals must be entered into the database, with the following exceptions:
- Biological Agents that are not toxins
- Biochemical materials such as cell-culture media, amino acids, or lipids that have been determined to be nonhazardous using available health and safety information
- Chemicals or chemical products transferred to secondary/split/child (nonmanufacturer) containers, if the primary container is accounted for accurately in CMS in terms of total quantity and location (e.g. room), but need to be labeled with content and primary hazard(s) at the minimum. These are jars, cans, squeeze bottles, and other containers to which hazardous materials are transferred from the original container by an individual. NOTE: Tracking splits of high hazard chemicals (under a new container record for the date of split created) is recommended as a best practice, if stored, to ensure they are accounted for if the primary container is disposed of in CMS. Tracking splits of time-sensitive chemicals, including peroxide-forming chemicals, is required, if stored, to ensure that time-sensitive hazards are mitigated. Process Guidance for Splits
- 🚩Samples such as research-produced chemicals and mixtures (follow labeling requirements in Work Process Y (Process guidance for samples)🛑
- Radiological materials that are tracked in RADAR
- Chemicals transferred to waste container
- Individual components of prepackaged chemical kits (see below)
- Consumer products, which can be excluded from the CMS if their usage is consistent with how the manufacturer intends the average consumer to use the product. Example: Bleach that is used infrequently for cleaning can be excluded. However, bleach that is used daily for work tasks such as disinfecting work surfaces in a tissue-culture laboratory is beyond what is considered average consumer usage as intended by the manufacturer and must be entered into CMS.
- All consumer adhesives and sealants must be inventoried.
- All gas cylinders shall be inventoried.
- Mixtures. The product name should be entered in CMS and the components and their concentration should be specified in the container comment field unless the information is included in the product name. It is recommended that you attach the Safety Data Sheet of the mixture to the container record in CMS. Examples: “nitric acid 65%” as the product name, “Nickel Standard for ICP” as the product name with container comment “nickel(II) nitrate 0.1%-1% and nitric acid 3%-5%”, “25PPM Hydrogen Sulfide, 100PPM Carbon Monoxide, 2.5% Methane, 18% Oxygen Balance Nitrogen Certified Standard Mixture“ as the product name.
- Consumable materials such as grinding wheels, welding rods, or solder material that can be used up, dispersed, or aerosolized during use must be entered into the CMS.
- Prepackaged kits that are sets of chemical components intended for use for a specific task, test, or procedure, must be entered into the CMS. NOTE: Individual components do not need to be entered individually. The kit may be entered as a whole, identified by the name as it appears on the manufacturer’s SDS.
- Multi-container Data Sheets/Static RFID Tag (Template 1 / Template 2, How-To Website)
- These can be used for groups of identical containers within a specific room in lieu of RFID tagging individual items. These sheets must be posted at or near the storage location. The number of containers represented by the RFID tag on a multi-container data sheet must be clearly identified. If the number of identical containers varies, a max amount possible should be identified (and should not be exceeded at any time). List the total quantity of all containers not to be exceeded in CMS (see examples, iii).
- Exceptions:
- Static RFID tags cannot be used for toxic or corrosive gas cylinders (e.g. NFPA Health Hazard Class 3 and 4, or Class 2 with poor warning properties) to enable accurate tracking.
- California and DOE regulations on the use of greenhouse gases require Berkeley Lab to report amounts annually. This multi-container option does not apply to greenhouse gases such as sulfur hexafluoride (SF6). All greenhouse gases such as SF6 must be individually entered into the CMS to enable accurate reporting.
- Static RFID tags for time-sensitive chemical solvents such as tetrahydrofuran and diethyl ether are not recommended to ensure containers are not lost or forgotten and accurate CMS tracking of container age.
- Examples of multi-container data sheets in use:
- All hazardous materials/chemicals must be entered into the database, with the following exceptions:
| Situation | Multi-container Data Sheet |
|
Gas cylinder storage area with:
| Sheet is posted clearly above gas storage racks:
|
|
Flammables storage cabinet containing:
|
Sheet is posted on the outside of the flammables cabinet:
|
|
Advanced Usage!
|
Two separate sheets with two separate RFID tags are posted:
Using this method, one cylinder can be moved from Room 100 to Room 200 and back as often as needed without the need to update CMS to reflect the change in room. |
h. Accessing CMS:
- CMS can be accessed at cms2.lbl.gov using your LDAP (same as email) username and password.
- Please contact CMS Support at CMS@lbl.gov for database access, editing privileges, setting up owner and rooms, and information regarding roles and responsibilities.
Work Process E. Chemical Hazard Evaluation: Identification, Classification, and Categorization
- Purpose of Chemical Hazard Evaluations
- Chemical hazard evaluations are conducted at LBNL to:
- Identify hazards of chemicals that are used in work activities for establishing engineering, work practice, administrative and training controls. These are conducted by Activity Leads and safety professionals for developing WPC Activities and for providing consultation to the Laboratory community. Refer to Work Process F.
- Classify and categorize the hazards of chemicals produced in laboratories to comply with Cal/OSHA Hazard Communication requirements.
- The Cal/OSHA Hazard Communication Standard (HCS) requires chemical manufacturers, importers and distributors to identify, classify and categorize the hazards of chemicals they produce. LBNL is regarded as a chemical manufacturer when chemicals produced in laboratories are shipped off site, and therefore must follow the hazard classification process prescribed by Cal/OSHA. Section 2 outlines these requirements. (Note: Due to the complex and prescriptive requirements for classifying chemicals, and for developing SDSs and labels under Cal/OSHA, researchers may consult with the CHSP manager for assistance.) In addition, LBNL must communicate these hazards to downstream users in the form of SDSs and labels. Refer to Work Process Z for additional details and hazard communication requirements for chemicals produced in laboratories.
- Chemical hazard evaluations are conducted at LBNL to:
- Chemical Hazard Definition, Classification and Categorization
- A hazardous chemical is any chemical classified as a health hazard or a physical hazard:
- Health Hazard: is a chemical posing one of the following hazardous effects: acute toxicity (any route of exposure); skin corrosion or irritation; serious eye damage or eye irritation; respiratory or skin sensitization; germ cell mutagenicity; carcinogenicity; reproductive toxicity; specific target organ toxicity (single or repeated exposure); or aspiration hazard.)
- Physical Hazard: is a chemical posing one of the following hazardous effects: explosive; flammable (gases, aerosols, liquids, or solids); oxidizer (liquid, solid or gas); self-reactive; pyrophoric (liquid or solid); self-heating; organic peroxide; corrosive to metal; gas under pressure; or in contact with water emits flammable gas.
- Cal/OSHA Hazard Classification and Categorization Process (Required for Chemical Manufacturers)
- Hazard classification is the process of evaluating available scientific evidence to determine if a chemical is hazardous, as well as to identify the level of severity of the hazardous effect. A hazard class is the type of health or physical hazard (e.g., acute toxicity and flammable liquid). A hazard category is the level of severity within each hazard class. When complete, the evaluation identifies the hazard class and hazard category of the chemical. This information is then used to develop SDSs and labels in order to communicate hazards to downstream users.
- The specific criteria for determining the hazard class and category are outlined in Appendices A and B of the HCS. Appendix A provides the classification criteria for health hazards and Appendix B provides the classification criteria for physical hazards. The OSHA publication, Hazard Communication: Hazard Classification Guidance for Manufacturers, Importers, and Employers, provides guidance for carrying out hazard classification.
- Testing of chemicals is not required by Cal/OSHA, but the full range of available scientific literature and other evidence concerning the potential hazards of chemicals to be considered in classifying chemical hazards must be considered.
- Due to the complex and prescriptive requirements for classifying chemicals, and for developing SDSs and labels under Cal/OSHA, researchers may consult with the CHSP manager for assistance.
- A hazardous chemical is any chemical classified as a health hazard or a physical hazard:
Note: LBNL is not required to use the process described above for routine hazard identification and evaluation conducted by Activity Leads and safety professionals in developing WPC Activities and providing consultation for work involving commercially procured chemicals.
- Information and Resources for Identifying and Evaluating Chemical Hazards. The following sources of information may be used to identify, classify and assess the hazards of chemicals. This is not an exhaustive list:
- The EH&S Safety Data Sheets and Chemical Information Resources site. This has several links to SDS databases and other toxicological and hazard resources.
- The Work Processes of the CHSP, in particular, Work Processes L-T (Specific Controls and Procedures).
- 8 CCR 5155, Airborne Contaminants, Table AC-1 has a list of hazardous chemicals and their respective airborne permissible exposure limits.
- Threshold Limit Values for Chemical Substances and Physical Agents in the Work Environment, American Conference of Governmental Industrial Hygienists (ACGIH), latest edition: Includes a list of hazardous chemicals and their respective airborne occupational exposure limits.
- International Agency for Research on Cancer (IARC): Evaluates the carcinogenicity of chemicals and ranks their carcinogenic potential. Chemicals with IARC classifications of 1, 2A, and 2B are considered by Cal/OSHA to be carcinogenic.
- National Toxicology Program (NTP) biennial Report on Carcinogens, latest edition: Chemicals with NTP classifications of A or B are considered by Cal/OSHA to be carcinogenic.
- National Institute for Occupational Safety and Health (NIOSH) Pocket Guide to Chemical Hazards: Presents the health and physical hazards of chemicals.
- The Registry of Toxic Effects of Chemical Substances (RTECS), National Institute for Occupational Safety and Health NIOSH (latest edition)
- Bretherick’s Handbook of Chemical Reactive Hazards
- Prudent Practices in the Laboratory: Handling and Management of Chemical Hazards, Updated Version.
Work Process F. Use and Application of Chemical Hazard Assessments
- Chemical hazard assessments are conducted to identify the hazards and necessary controls for chemicals used in shop, field, and laboratory work environments. The inherent chemical and physical hazards (i.e., hazard class and category) as well as the manner in which chemicals are used, are considered. Specifically, hazard assessments are performed to:
- Develop Work Planning and Control (WPC) activities – WPC is a review and management-approval process designed to ensure that procedures, controls, and resources are in place before work begins. Activity Leads develop activities by defining work, identifying hazards associated with the work, and implementing controls. The Activity Lead is also responsible for assigning and authorizing workers to perform work. Consult the Work Planning and Control program (EH&S Manual Chapter 6) for additional details and requirements.
- Perform Exposure Assessments – EHS Health and Safety Representatives identify the potential for exposure to chemicals and provide options for minimizing risk through hazard elimination, engineering controls, personal protective equipment (PPE), and administrative controls. These assessments are generally qualitative, although quantitative exposure assessments (collection and analysis of air and bulk samples) may be performed.
- Perform respiratory protection evaluations – EHS Health and Safety Representatives evaluate chemical usage and work activities for recommending respiratory protection.
- In accordance with Integrated Safety Management (ISM) Activity Leads and Project Leads are responsible for “integrating EHS into work and for ensuring active communication up and down the management line with the workforce.” To this end, they must be aware of and authorize the work performed under their jurisdiction and ensure that appropriate hazard assessments and exposure assessments, as necessary, are conducted in their work areas.
- Laboratory and technical area safety surveys will be performed by EHS in each lab and technical area at least once every two years to assure chemical hazard and exposure assessments are conducted as needed. During the surveys, work scope will be discussed and safety of the areas will be evaluated.
- All work performed at Berkeley Lab must be authorized through WPC or an equivalent work authorization process (e.g. User authorization processes at the ALS or the Molecular Foundry). The Laboratory protocol for determining the proper level of work authorization is delineated in the Pub 3000 Chapter 6, Safe Work Authorizations program. The ultimate determination of the appropriate work authorization is the responsibility of the Project Lead (with approval from a worker’s manager/supervisor for Risk Level 3 activities) for that work.
- The Cal/OSHA Laboratory Standard requires that employers identify higher hazard work that requires prior approval. At LBNL, that is accomplished through WPC. With regard to chemicals, three WPC risk levels were established:
- Risk level 1 chemicals are those routinely found in most labs. They include materials such as flammable/combustible liquids, acids and bases, where the use of standard safe laboratory practices and controls such as fume hoods, proper storage, housekeeping and PPE can adequately control the hazards.
- Risk level 2 chemicals are also common but they have a higher degree of inherent hazard and require more specialized controls. These include materials such as Cal/OSHA “Particularly Hazardous Substances” (e.g., reproductive toxins and carcinogens), peroxide formers, and phenol. Risk level 1 controls (fume hood, PPE, etc.) apply to these materials, but due to their nature, additional controls are required. For example, particularly hazardous substances need to be used in “designated areas”, peroxide formers require labeling and testing and emergency exposure kits are needed for phenol.
- Risk level 3 chemicals have the highest degree of hazard, in which a loss of control could result in immediate injury or death, and therefore require a higher level of control and authorization. These include water reactives, pyrophorics, lethal toxicants, and secondary explosives. Below are several lists of chemicals that can trigger risk level 3 activities. Note these lists are NOT exhaustive. Other sources such as those listed in Work Process E may be consulted. An EHS Health and Safety Representative may also be consulted.
- Toxic/Pyrophoric Gases
| 1,3-butadiene | iodine pentafluoride |
| ammonia | methyl bromide |
| arsenic pentafluoride | methyl chloride |
| arsine | methyl silane |
| boron trichloride | nickel carbonyl |
| boron trifluoride | nitric oxide |
| bromine pentafluoride | nitrogen dioxide |
| bromine trifluoride | nitrogen trifluoride |
| carbon monoxide | nitrosyl chloride |
| carbonyl fluoride | oxygen difluoride |
| carbonyl sulfide | phosgene |
| chlorine | phosphine |
| chlorine trifluoride | phosphorus pentafluoride |
| cyanogen | phosphorus trichloride |
| cyanogen chloride | phosphorus trifluoride |
| diborane | selenium hexafluoride |
| dichlorosilane | silane |
| fluorine | silicon tetrafluoride |
| germane | stibine |
| hydrogen bromide | sulfur dioxide |
| hydrogen chloride | sulfur tetrafluoride |
| hydrogen cyanide | sulfuryl fluoride |
| hydrogen fluoride | tellurium hexafluoride |
| hydrogen selenide | tungsten hexafluoride |
| hydrogen sulfide | vinyl chloride |
- Reactive and Explosive Substances
| aluminum hydride | picric acid |
| benzoyl peroxide | potassium |
| cesium | rubidium |
| copper azide | sodium |
| lead azide | sodium azide |
| lithium | triethyl aluminum |
| phosphorus (white) | trimethyl aluminum |
- Chemicals Possessing Lethal or Incapacitating Toxicity
| Amiton: O,O-Diethyl S-[2-(diethylamino)ethyl] phosphorothiolate and corresponding alkylated or protonated salts (78-53-5) |
| O-Alkyl (<C10, incl. cycloalkyl) alkyl (Me, Et, n-Pr or i-Pr)-phosphonofluoridates Sarin: O-Isopropyl methylphosphonofluoridate (107-44-8) Soman: O-Pinacolyl methylphosphonofluoridate (96-64-0) |
| O-Alkyl (<C10, incl. cycloalkyl) N,N-dialkyl (Me, Et, n-Pr or i-Pr) phosphoramidocyanidates Tabun: O-Ethyl N,N-dimethyl phosphoramidocyanidate (77-81-6) |
| O-Alkyl (H or <C10, incl. cycloalkyl) S-2-dialkyl (Me, Et, n-Pr or i-Pr)-aminoethyl alkyl(Me, Et, n-Pr or i-Pr) phosphonothiolates and corresponding alkylated or protonated salts VX: O-Ethyl S-2-diisopropylaminoethyl methyl phosphonothiolate [50782-69-9] |
| Sulfur mustards: 2-Chloroethylchloromethylsulfide [2625-76-5] Mustard gas: Bis(2-chloroethyl)sulfide [505-60-2] Bis(2-chloroethylthio)methane [63869-13-6] Sesquimustard: 1,2-Bis(2-chloroethylthio)ethane [3563-36-8] 1,3-Bis(2-chloroethylthio)-n-propane [63905-10-2] 1,4-Bis(2-chloroethylthio)-n-butane [142868-93-7] 1,5-Bis(2-chloroethylthio)-n-pentane [142868-94-8] Bis(2-chloroethylthiomethyl)ether [63918-90-1] O-Mustard: Bis(2-chloroethylthioethyl)ether [63918-89-8] |
| Lewisites: Lewisite 1: 2-Chlorovinyldichloroarsine [541-25-3] Lewisite 2: Bis(2-chlorovinyl)chloroarsine [40334-69-8] Lewisite 3: Tris(2-chlorovinyl)arsine [40334-70-1] |
| Nitrogen mustards: HN1: Bis(2-chloroethyl)ethylamine [538-07-8] HN2: Bis(2-chloroethyl)methylamine [51-75-2] HN3: Tris(2-chloroethyl)amine [555-77-1] |
| Saxitoxin [35523-89-8] |
| Ricin [9009-86-3] |
| Alkyl (Me, Et, n-Pr or i-Pr) phosphonyldifluorides DF: Methylphosphonyldifluoride [676-99-3] |
| O-Alkyl (H or <C10, incl. cycloalkyl) O-2-dialkyl(Me, Et, n-Pr or i-Pr)-aminoethyl alkyl(Me, Et, n-Pr or i-Pr) phosphonites and corresponding alkylated or protonated salts QL: O-Ethyl O-2-diisopropylaminoethyl methylphosphonite [57856-11-8] |
| Chlorosarin: O-Isopropyl methylphosphonochloridate [1445-76-7] |
| Chlorosoman: O-Pinacolyl methylphosphonochloridate [7040-57-5] |
| PFIB: 1,1,3,3,3-Pentafluoro-2-(trifluoromethyl)-1-propene [382-21-8] |
| BZ: 3-Quinuclidinyl benzilate (*) [6581-06-2] |
| Chemicals containing a phosphorus atom to which is bonded one methyl, ethyl, or propyl (normal or iso) group but no further carbon atoms Methylphosphonyl dichloride [676-97-1] Dimethyl methylphosphonate [756-79-6] Exemption: Fonofos: O-Ethyl S-phenyl ethylphosphonothiolothionate [944-22-9] |
| N,N-Dialkyl (Me, Et, n-Pr or i-Pr) phosphoramidic dihalides |
| Dialkyl (Me, Et, n-Pr or i-Pr) N,N-dialkyl(Me, Et, n-Pr or i-Pr)-phosphoramidates |
| Arsenic trichloride [7784-34-1] |
| 2,2-Diphenyl-2-hydroxyacetic acid [76-93-7] |
| Quinuclidin-3-ol [1619-34-7] |
| N,N-Dialkyl (Me, Et, n-Pr or i-Pr) aminoethyl-2-chlorides and corresponding protonated salts |
| N,N-Dialkyl (Me, Et, n-Pr or i-Pr) aminoethane-2-ols and corresponding protonated salts Exemptions: N,N-Dimethylaminoethanol and corresponding protonated salts [108-01-0] N,N-Diethylaminoethanol and corresponding protonated salts [100-37-8] |
| N,N-Dialkyl (Me, Et, n-Pr or i-Pr) aminoethane-2-thiols and corresponding protonated salts |
| Thiodiglycol: Bis(2-hydroxyethyl)sulfide [111-48-8] |
| Pinacolyl alcohol: 3,3-Dimethylbutan-2-ol [464-07-3] |
- The following chemicals also exhibit toxic properties of concern. An EHS Health and Safety Representatives must be consulted prior to purchase and use of these chemicals to determine if additional work authorization or approvals are required:
| Chemical Name [CAS#] |
| Phosgene: Carbonyl dichloride [75-44-5] |
| Chloropicrin: Trichloronitromethane [76-06-2] |
| Phosphorus oxychloride [10025-87-3] |
| Phosphorus trichloride [7719-12-2] |
| Phosphorus pentachloride [10026-13-8] |
| Trimethyl phosphite [121-45-9] |
| Triethyl phosphite [122-52-1] |
| Dimethyl phosphite [868-85-9] |
| Diethyl phosphite [762-04-9] |
| Sulfur monochloride [10025-67-9] |
| Sulfur dichloride [10545-99-0] |
| Thionyl chloride [7719-09-7] |
| Ethyldiethanolamine [139-87-7] |
| Methyldiethanolamine [105-59-9] |
| Triethanolamine [102-71-6] |
Work Process G. General Controls for Hazardous Chemicals
This work process discusses control procedures for limiting employee exposure to chemical hazards.
- Standard Operating Procedures. Standard operating procedures for all chemicals at Berkeley Lab are intended to minimize employee exposure to hazards, and include: chemical substitution, engineering controls, administrative controls, personal protective equipment, work practice controls, and emergency procedures, all of which are described in this section. All employees are required to wear eye protection, lab coats, and chemically resistant gloves when handling hazardous chemicals. Operations that may generate airborne gases, vapors, dusts, fumes, and smoke must be done in a fume hood or glove box. In addition, specific controls for the following classes of hazardous materials are described in more detail below. Activity Leads shall ensure that all work involving these hazardous chemicals is evaluated, controlled, documented, and approved in a WPC Activity.
- Acids and Bases (Work Process L)
- Particularly Hazardous Substances (Work Process M)
- Carcinogens (Work Process M.1)
- Reproductive Toxins (Work Process M.2)
- Flammables and Combustible Liquids (Work Process N)
- Laser Dyes and Solvents (Work Process O)
- Peroxide-Forming Compounds (Work Process P)
- Water-Reactive Chemicals (Work Process Q)
- Pyrophoric Materials (Work Process R)
- Chemical Synthesis (Work Process R.1)
- Engineered Nanomaterials (Work Process S)
- Chemicals with Explosive Properties (Work Process T)
Chemical Purchasing
- Determine whether the chemical is a restricted item. If it is, the person making the decision to purchase the chemical or giving permission to another institution to have it delivered to Berkeley Lab must ensure notification or approval is obtained from the EHS Division in accordance with the Laboratory’s procurement requirements. Refer to Work Process B for additional details.
- Determine whether the chemical is a restricted item. If it is, the person making the decision to purchase the chemical or giving permission to another institution to have it delivered to Berkeley Lab must ensure notification or approval is obtained from the EHS Division in accordance with the Laboratory’s procurement requirements. Refer to Work Process B for additional details.
- Chemical Use, Selection, and Substitution. Before a chemical or a product is introduced or used in a workplace, the Activity Lead must:
- Review the hazards of the material and assess the conditions under which it will be used. This information may be obtained from the SDS or by consultation with EHS Health and Safety Representatives. Work Process E lists information and resources for identifying and evaluating chemical hazards.
- Determine whether the chemical can be substituted with a safer chemical alternative. An EHS Health and Safety Representatives can be consulted to provide assistance to identify substitute chemicals.
- Determine whether the chemical can be borrowed from someone within the research group or the division. If the chemical must be purchased, keep working quantities of all hazardous materials to a minimum. Procure, use, and store the minimum amount of material required.
- Chemicals Produced in Laboratories. Chemicals produced in laboratories must, at a minimum, be evaluated for their hazards. Other requirements such as training, and the production of SDSs and labels vary depending on whether the materials will be shipped off site or used on-site. Refer to Work Process Z for additional details and hazard communication requirements for chemicals produced in laboratories.
Work Process H. Selection and Use of Engineering Controls
Engineering controls include local exhaust ventilation systems, laboratory fume hoods, enclosures, and shields. Except for substitution, these provide the most effective means of control because they enclose the hazard or physically separate it from the employee.
- Local Exhaust Ventilation, Fume Hoods
- Local exhaust ventilation and laboratory fume hoods are used to remove airborne contaminants from an employee’s breathing zone. Self-contained (ductless) hoods that recirculate air back into the workspace are not an acceptable means to control airborne chemicals. The selection, procurement, installation, and balancing of all ventilation systems must be done through Facilities.

- Local exhaust ventilation (such as laboratory fume hoods, glove boxes, extractor arms, or industrial ventilation) is required when handling chemicals in a manner that can produce an airborne hazard.

- Examples of activities requiring local exhaust ventilation include but are not limited to:
- Using particularly hazardous substances (i.e., acutely toxic, carcinogenic, or reproductive toxins)
- Handling volatile toxic liquids
- Using organic liquids or solvents
- Conducting procedures that generate airborne particulates (e.g., dust) or liquid aerosols of even moderately toxic chemicals
- Using odiferous compounds
- Synthesizing or reacting chemicals
- Diluting concentrated acids and bases. NOTE: Operations involving heating or evaporating perchloric acid must be evaluated by an EHS Health and Safety Representative to determine whether special controls (such as using an acid fume hood with wash-down systems to prevent the accumulation of explosive perchlorate crystals) are needed
- Discharging gases/vapors from vacuum pumps and distillation columns
- Discharging harmful gases and vapors from drying ovens and muffle furnaces; NOTE: Consult an EHS Health and Safety Representative to help make this determination.
- Performing operations that could generate a flammable atmosphere
- Transfers of flammable and combustible liquids from one container to another. Refer to Work Process N.11, “Transfer Operations,” for specific guidance and requirements regarding these transfers.
- Fume hoods, gas cabinets, and other ventilated enclosures must be equipped with electronic flow sensors or pressure gauges. If the flow sensor alarm goes off (red light, warning sound) or the pressure falls below the indicated set point on the pressure gauge, discontinue work in that system and call the Work Request Center (ext. 6274; for after-hours emergency service, call ext. 5481) to report the condition. Do not simply mute the alarm and continue working — the alarm indicates that something is not operating correctly. If the system is restored and the alarm resets, you may resume work, but you should report the condition so that the cause may be investigated.
- Local exhaust ventilation and laboratory fume hoods are used to remove airborne contaminants from an employee’s breathing zone. Self-contained (ductless) hoods that recirculate air back into the workspace are not an acceptable means to control airborne chemicals. The selection, procurement, installation, and balancing of all ventilation systems must be done through Facilities.
- Glove Boxes and Gas Cabinets
- Glove boxes that provide a nonreactive atmosphere are required for operations involving alkali metals and pyrophoric materials. Fume hoods may also be used provided that measures are taken to prevent control moisture and air, such
- Gas cabinets are required for health-hazard and pyrophoric gases as described in ES&H Manual Chapter 13 Gas Safety program. NOTE: Fume hoods may be used for this purpose as well, based on review and concurrence from an EHS Health and Safety Representative.
- Chemical Storage Cabinets
- New flammable storage cabinets must be connected to the building’s exhaust system. This applies to all flammable storage cabinets installed during renovation or new construction. Refer to Work Process N for additional requirements for flammable storage cabinets.
- Existing flammable storage cabinets showing signs of interior corrosion or whose contents produce strong odors during storage shall also be ventilated.
- It is recommended that new corrosive storage cabinets be connected to the building’s exhaust system. Existing cabinets may also need to be connected if they show signs of corrosion or produce odors. Consult with your Division’s Health and Safety Representative for guidance.
- Laboratory Room Exhaust. Laboratory heating, ventilating, and air conditioning (HVAC) systems must provide 100% outside air to laboratory spaces (no recirculation of air is allowed). The HVAC systems are balanced to keep laboratory spaces at a negative pressure relative to adjacent offices and hallways. This ensures that vapors, gases, fumes, and particulates do not migrate to non-laboratory spaces. A minimum ventilation rate of 1 cubic foot per minute of exhaust per square foot of laboratory area is required. Exceptions to maintaining negative pressure in a laboratory can be made provided that it can be demonstrated that research is adversely affected by ambient air drawn into the workspace and that EHS Health and Safety Representative concurrence has been obtained.
- Ventilation System Performance Evaluations
- Facilities is responsible for installing, balancing, and function testing all ventilation devices.
- EHS Health and Safety Representatives perform periodic testing of fume hoods, gas cabinets, glove boxes, canopy hoods, and extractor arms. These are evaluated initially after installation. Fume hoods used for radiological work are evaluated annually, while fume hoods used for non-radiological work are evaluated every two years (with an annual spot check in between formal evaluations). All other ventilation units – gas cabinets, glove boxes, canopies, and extractor arms – are evaluated annually.
- Ventilation system performance must be checked whenever the system has been modified, such as by adding new hoods or relocating or replacing system components (including hoods).

- Safety Shields. Safety shields must be used for protection against possible explosions or uncontrolled reactions. Laboratory equipment must be shielded on all sides to ensure there is no line-of-sight exposure of personnel.
Work Process I. Personal Protective Equipment
Personal protective equipment (PPE) is to be used as a supplement to but not as a substitute for engineering controls. PPE includes chemically resistant gloves, eyewear, footwear, lab coats, aprons, coveralls, and respiratory protection. PPE may be used as a sole means of control if the use of other controls is not feasible. PPE is provided at no personal expense to the individual. To be effective, employees must understand the proper selection, use, and limitations of PPE. For additional information on Berkeley Lab’s policies on PPE, see the ES&H Manual Personal Protective Equipment program.
- General PPE Requirements
- Skin and eye contact must be prevented. PPE must be selected on the basis of the hazards present, the type of materials used, and the manner in which they will be handled. Activity leads are responsible for ensuring that PPE use is included in WPC activities and for ensuring that employees use PPE properly.
- Employees must be trained in the uses and limitations of PPE. This is the activity lead’s responsibility. An EHS Health and Safety Representative may be consulted to provide guidance on the selection and use of PPE and to assist in training.
- Employees must report problems (such as deterioration and degradation) to activity leads immediately.

- Removing PPE
- Remove lab coats, coveralls, and gloves prior to leaving technical areas (labs, workrooms, and similar areas) when going to common areas such as lunchrooms, conference rooms, offices, restrooms, and the cafeteria.
- Remove gloves before touching common use items such as phones, computers, light switches, and doorknobs.
- Remove lab coats, coveralls, and gloves used for protection against engineered nanomaterials before leaving the area, regardless of your destination.
- The above requirements also apply to technical areas where biological materials are used.
- Disposing of PPE
- Normally, disposable PPE used in laboratory settings (such as gloves, Tyvek coveralls, and booties) may be disposed of as regular trash. However, PPE used for protection against materials such as engineered nanomaterials, lead, and asbestos must be disposed of as hazardous waste. Consult with an EHS Waste Generator Assistant for more information.
- Consult with a radiological control technician for disposing of PPE used in radiological areas.
- Dispose of PPE used for protection against biological materials in accordance with Berkeley Lab medical waste management procedures.
- Minimum PPE Requirements
- Area PPE Requirements
- Area PPE requirements must be established for all technical areas. This is the responsibility of the area safety leader through consultation with supervisors and activity leads.
- Area PPE requirements must be listed on the Berkeley Lab entrance placard.
- Area PPE requirements apply to the entire technical area unless an exception is granted in accordance with the procedure described in Appendix A, PPE and Food/Drink Requirements and Responsibilities Table, of the Personal Protective Equipment (PPE) program in the ES&H Manual.
- Minimum Area PPE requirements for technical areas are as follows (this applies to visitors as well as Lab personnel):
- Safety glasses with side shields
- Closed-toe shoes
- Long pants
- Area PPE Requirements
- Gloves
- Gloves must be worn when using hazardous chemicals, when handling materials at temperature extremes, or when handling materials with sharp or rough surfaces. It is especially important to wear gloves when handling chemicals that can be absorbed through the intact skin. Consult SDSs and the OSHA Occupational Chemical Database to identify chemicals that have this property. This may not be a complete list; therefore, contact an EHS Health and Safety Representative if you have any questions about a particular compound.
- Store gloves in a clean area outside of fume hoods and away from equipment that could potentially contaminate them.
- Always remove gloves before touching common use items such as phones, doorknobs, and computers. This will prevent contamination of unprotected individuals.
- Glove Selection
- Chemically resistant gloves are manufactured from a variety of materials, including nitrile, polyvinyl chloride, natural rubber (latex), and Viton. No single glove material provides universal protection against all chemical agents. Therefore, gloves must be selected on the basis of their resistance to the material(s) being handled, their suitability for the procedures being conducted, and their resistance to wear as well as temperature extremes.
- Improper selection may degrade the gloves, allow the chemical to permeate through the gloves, and ultimately expose the wearer to the chemical. This is a potentially serious situation. Use chemical resistance charts and glove selection databases (below) to choose gloves.
- In addition to the specific chemical(s), other factors to consider in selecting gloves are how and where they will be used. In shop environments, gloves may be subjected to rougher handling and may be totally immersed in chemicals such as cleaners and degreasers. In labs, manual dexterity may be an issue; splashes, as opposed to total immersion in the chemical, are more common. Gloves used in shop settings are thus required to be more resistant to tears and abrasion than those used in laboratory environments and are normally thicker (greater than 10-15 mils). In laboratories, thin, lightweight gloves are generally preferred (less than 10 mils). As a point of reference, typical dishwashing gloves are approximately 15 to 20 mils thick, and surgical latex gloves are on the order of 3 to 8 mils thick.
- A final consideration in glove selection is an individual’s sensitivity to the materials and chemicals used in the manufacture of gloves. Some people have allergic reactions to natural rubber proteins in latex, glove powder (used for absorbing perspiration), or other chemical constituents, such as rubber accelerators (carbamates, thiurams, and mercaptobenzothiazole).
- Chemical-Resistance Charts and Glove-Selection Databases
- Objective data (such as chemical-resistance charts and selection databases) must be used to choose the appropriate glove. Chemical-resistance charts are available from the CHSP manager (or directly from the manufacturer).
- The Web sites listed below are recommended for selecting chemically resistant gloves. Some allow different ways to search for the most appropriate glove. Searching by chemical name will produce a list of gloves that protect against a particular agent. Searching by glove type will give a list of chemicals for which a specific glove was tested. Glove thickness is normally listed or is available by clicking on the link for the recommended glove.
- Glove Manufacturer Web Sites:
- Independent Glove Selection Web Sites:
- Michigan State University Chemical-Resistant Glove Guide
- Oklahoma State University Chemical Guide and Permeation Tables
- An EHS Health and Safety Representative may also be contacted for assistance in glove selection.
- Eye and Face Protection. This section discusses the uses and limitations of safety glasses, chemical goggles, and face shields. The type of protection selected must be based on risk — i.e., the degree of hazard (severity) and the likelihood of an accident occurring (probability).

- All eye and face protection must meet the Basic Impact Testing Requirements of the latest version of the American National Standard for Occupational and Educational Personal Eye and Face Protection Devices ANSI Z87.1. (These eye-protection devices are marked “Z87.”)
- Where there is a possibility of a hazard from flying particles, the eye protection must meet the High Impact Testing Requirements of Z87.1 (this eye protection is marked “Z87+”).
- An EHS Health and Safety Representative may be consulted to provide guidance in selecting the proper protection.
- Safety Glasses
- Safety glasses with side shields are required to be worn by individuals while handling or using chemicals. They must meet the basic impact-resistance provisions of ANSI Z87.1.
- Safety glasses are the minimum protection required when handling chemicals. Safety glasses must be supplemented with goggles and/or face shields when there is a greater risk of exposure to chemical splashes or flying particles (e.g., when pouring or mixing chemicals or cryogens).
- Contact lenses may be worn in work areas. However, contact lenses do not provide eye protection. Safety glasses or goggles must be worn by people who use contact lenses when chemicals are being handled.
- Cover Goggles
- Cover goggles are required for operations where there is a greater risk of exposure to chemicals and to flying particles. Furthermore, they are required for activities producing airborne eye irritants including gases, vapors, fumes, dusts, and mists. Safety glasses provide no protection against eye irritants.
- Cover goggles are available in several varieties including:
- Direct-vented. These allow airflow for comfort and to reduce fogging. Generally, direct-vented goggles are inappropriate for liquid chemical use because the vent ports may allow splashed liquids to pass through. Moreover, they provide no protection against airborne eye irritants (gases, vapors, fumes, dusts, and mists).
- Indirect-vented. These allow airflow for comfort and to reduce fogging. These are appropriate for liquid chemical use and will protect the eyes from splashes. However they will not guard against irritating gases, vapors, fumes, dusts, and mists.
- Non-vented. These have no vents and are required for operations that produce airborne irritating gases, vapors, fumes, dusts, and mists. They also protect against chemical splashes to the eyes.
- Face Shields
- Face shields protect the entire face and are required for operations that present a high likelihood of exposure to flying particles or splashes from liquid chemicals or cryogenic fluids.
- Face shields must be worn in conjunction with safety glasses or chemical goggles, because face shields can be lifted up during use, exposing the eyes to hazards.
- Respirators
- Respirators should not be needed in most laboratory and shop settings. However, if engineering, work practices, and administrative controls are not adequate to minimize an airborne chemical hazard, respiratory protection is required.
- Use of respirators requires a hazard evaluation conducted by an EHS Health and Safety Representative. All respirator users must be medically qualified, trained, and fit-tested to wear respiratory protection equipment.
- An EHS Health and Safety Representative must approve procurement of respirators.
- Any questions regarding the need for or use of respirators should be directed to an EHS Health and Safety Representative. Berkeley Lab respirator policy may be found in the EH&S Chapter 44, Respiratory Protection program.
Work Process J. Work Practice Controls
Work practice controls include pre-planning work, practicing good housekeeping, maintaining personal hygiene to minimize exposure to hazardous materials, and using common sense. Work practice controls must be used regardless of the type of hazardous material handled. This is not accomplished through the WPC process alone. This evaluation should be performed prior to the start of any task. The first three steps of the Integrated Safety Management (ISM) process should be employed prior to the initiation of work: Define the scope of your work; Identify all hazards associated with the work; Identify the controls necessary to mitigate those hazards.
- Work Planning
- Pre-plan work: Stage tools, equipment, and materials in advance of the activity to be performed.
- Establish designated areas for work involving particularly hazardous substances.
- Stay upwind or use exhaust ventilation for operations that emit vapors, gases, fumes, dusts, mists, or aerosols.
- Limit the amount of hazardous materials procured, used, and stored to the minimum needed for an operation.
- Keep drip pans, secondary containment and cleanup materials readily available.
- Be familiar with the use, limitations, and location of emergency equipment such as emergency eyewashes, safety showers, fire alarms, exits, and fire extinguishers.
- Keep containers covered when not being used.
- Remove jewelry to prevent contact with electrical sources and chemicals and from catching on laboratory or shop equipment.
- Confine long hair and loose clothing when working in the laboratory/shop.
- Housekeeping
- Keep work areas clean and free of obstructions. Clean the work area at the completion of an operation or at the end of the day.
- Wipe drips and residues from containers of hazardous materials. Skin contact with residues may cause dermal absorption, chemical burns, skin irritation, and possible accidental ingestion as a result of hand-to-mouth transfer.
- Clean surfaces (counter tops, bench tops, fume hoods, and floors) of all drips and residues.
- Clean spilled chemicals immediately, and dispose of all wastes properly. Spill response is discussed later. Chemical wastes must be disposed of in accordance with the ES&H Manual Waste Management program.
- Maintain access to exits, emergency equipment, and other control equipment. Do not use stairways and hallways as storage areas. Store equipment and chemicals properly, and avoid clutter.
- Personal Hygiene
- After handling chemicals, wash hands with soap and water before leaving the laboratory/shop area and prior to breaks and consumption of food/beverages.
- Always remove gloves before touching common use items such as phones, doorknobs, and computers. This will prevent contamination of unprotected individuals.

- Food, Beverage, Cosmetics, and Medicine in Technical Areas
- Food and beverages (including water, gum, and medicines) may not be consumed or stored in technical areas. Exceptions may be granted in accordance with the procedure described in ES&H Manual Personal Protective Equipment (PPE) program, Appendix A, PPE and Food/Drink Requirements and Responsibilities Table.
- Cosmetics, ointments, skin cream, and similar items may not be applied or stored in technical areas.
- See Work Process K.1.b, Refrigerators and Freezers Used for Hazardous Material Storage, for requirements regarding food storage in refrigerators.
- Do not use laboratory glassware or utensils to prepare or consume food or beverages.
- Use of Glassware
- Never use mouth suction to pipette chemicals or to start a siphon; use mechanical means, a pipette bulb, or an aspirator.
- Use adequate hand protection (e.g., proper gloves) when inserting glass tubing into rubber stoppers or corks or when placing rubber tubing on glass hose connections. Tubing should be fire-polished or rounded and lubricated, and hands should be held close together to limit movement of glass should fracture occur. Plastic or metal connectors should be used whenever possible.
- Do not attempt glassblowing operations unless proper annealing facilities are available.
- Handle vacuum-jacketed glass apparatus with extreme care to prevent implosions. Equipment such as dewar flasks should be taped or shielded. Only glassware designed for vacuum work should be used for that purpose. Consult the ES&H Manual Chapter 7, Pressure Safety and Cryogenics program, for the safe use of pressurized and evacuated systems.
- Protect hands (i.e., wear tear- and puncture-resistant gloves) when picking up broken glass.
- Disposal of Glassware. Dispose of glass in marked cardboard boxes designated for that purpose. Glassware must be free of liquids prior to disposal. Consult Section 2.2, Solid Medical/Biohazardous Waste Disposal, of the Medical and Biohazardous Waste Generator’s Guide (PUB-3095) for guidance on disposing of glassware that has been used for biological work or that may be contaminated with a biohazardous material.

- Administrative Controls. Administrative controls include written procedures, employee training, establishing designated or restricted areas, chemical procurement procedures, and preventive maintenance. The development and use of written safety authorizations are discussed in the Work Planning and Control program (EH&S Manual Chapter 6). Training is discussed in Work Process W, Training, and Work Process X, Safety Data Sheets. The establishment and use of designated areas are discussed in Work Process M, Specific Controls and Procedures — Particularly Hazardous Substances: Carcinogens, Reproductive Toxins, and Acute Toxins.
Work Process K. Chemical Storage
This section provides requirements and recommendations for storing hazardous materials, 🚩whether in their original manufacturer containers, transferred into a new storage container, or contained in research-produced samples of hazardous materials and hazard categories.🛑 Refer to Work Process E, Chemical Hazard Evaluation: Identification, Classification, and Categorization, and Work Process F, Use and Application of Chemical Hazard Assessments, to determine whether a chemical, material, product, or mixture is hazardous. Refer to the ES&H Manual Chapter 20, Waste Management program for hazardous waste storage requirements.

- Hazardous Materials Storage Requirements. The criteria listed in this section are requirements that must be followed by Berkeley Lab staff.
- General Requirements, Storage Cabinets, and Shelves.
- Segregate incompatible chemicals (e.g., store oxidizing acids and flammable solvents in separate locations). This is to prevent inadvertent mixing of incompatible chemicals, which can produce harmful gases/vapors, heat, fire, and explosions. Consult specific controls and procedures described in Work Process L through Work Process T of this program for additional requirements and details tailored for various categories of hazardous materials. The chemical incompatibility matrices and tables presented later in this section provide recommended (optional) guidelines for segregating incompatible chemicals.
- Store hazardous materials away from heat and direct sunlight. Heat and sunlight may affect and degrade chemicals and deteriorate storage containers and labels.
- Do not store hazardous materials (except cleaners) under sinks.
- Ensure caps and lids are securely tightened on containers. This prevents leaks and evaporation of contents.
- Use approved flammable storage cabinets or flammable storage containers to store flammable and combustible liquids. Flammable and combustible liquids kept in squeeze bottles and other secondary containers may be kept on counter and bench tops provided they are in use or staged for use and are kept in secondary containment. Note: Storage of nonflammable solvents such as chloroform and methylene chloride are permitted in flammable storage cabinets provided that (1) they are chemically compatible with the other stored chemicals and (2) storage of non-flammables does not displace flammable and combustible chemicals from the storage cabinet. See Work Process N, Specific Controls and Procedures — Flammables and Combustible Liquids, for additional requirements and details on storage.
- Store inorganic acids in corrosive or acid storage cabinets. See Control Procedures for Acids and Bases for additional requirements. Their interiors and hardware (door hinges and shelf brackets) are corrosion resistant. Corrosive storage cabinets can be located under fume hoods or exist as stand-alone units. Flammable storage cabinets are not corrosion resistant and shall not be used for inorganic acid storage.
- Install Plexiglas lips or use equivalent means to prevent materials from falling off open storage shelves.
- 🚩Research-Produced Chemical/Mixture/Sample Storage Area Requirements
- The storage of research-produced hazardous materials and samples must follow the segregation and storage requirements per this Work Process. Lower-hazard (e.g. non-flammable/ignitable, non-corrosive, stable, low toxicity) and non-hazardous sample storage in areas such as regular cabinets, drawers, etc. must be identified at minimum with the label “Sample Storage” and words, pictures, symbols, or combination thereof to identify the hazards🛑
- Refrigerators and Freezers Used for Hazardous Material Storage
- Refrigerators and freezers, collectively referred to as “refrigerator/freezers,” used for storing flammable and combustible liquids shall be designed and labeled for that purpose. Do not use ordinary domestic units. See Work Process N, Specific Controls and Procedures — Flammables and Combustible Liquids, for additional safety requirements.
- Do not store food, beverages, or ice (intended for human consumption) in refrigerators/freezers located in technical areas.
- Refrigerators/freezers at LBNL must be labeled according to they type, purpose, and location. Refer to Table K-1 below.
- Squeeze Bottles and Wash Bottles. Hazardous materials are often transferred to squeeze bottles and other plastic containers such as NalgeneTM bottles. These are made of plastics such as high-density polyethylene, low-density polyethylene, and polypropylene and may exhibit varying degrees of resistance to chemicals. Moreover, they may deteriorate over time, especially when exposed to sunlight or UV sources. Utilize resources such as user knowledge or chemical-resistance data such as that provided by the Thermo Fisher Nalgene web site to determine and select the proper material.
- Secondary Containment
- Store liquid hazardous materials (including squeeze and wash bottles) in secondary containment. This is to minimize the impact and spread of spills resulting from broken/leaking containers. Secondary containment capacity must be 110% of the largest container or 10% of the aggregate volume of all containers, whichever is larger.
- Secondary containment is available in different materials that provide varying resistance to various chemicals. Use resources such as user knowledge or the information provided below to select the proper material.
- Photo Trays
- Generally, these provide good resistance to aqueous solutions and some organic solvents. But they may not be a good choice for halogenated solvents.
- Photo trays are available through several commercial sources, including VWR Scientific. An additional source of spill containment trays is Scientific Plastics. This company provides trays in several depths, with width and length in 1-inch increments. These trays have been used at Berkeley Lab to contain entire shelves in storage cabinets.
- Polypropylene and High-Density Polyethylene Trays
- These may be affected by some aromatic and halogenated hydrocarbons.
- The Thermo Fisher NalgeneTM web site has a chemical-resistance database for these materials.
- Stainless Steel and Pyrex Trays. Stainless steel and Pyrex trays are resistant to a broader spectrum of chemicals. However, they are more costly than plastic trays and are not available in as many different sizes and configurations.
- Larger-Capacity Containers. Containers such as Palletote® boxes are acceptable for larger volumes of liquids provided they are resistant to the chemicals stored in them. Palletote® boxes are constructed of high-density polyethylene.
- General Requirements, Storage Cabinets, and Shelves.
- Hazardous Material Storage Recommendations. The information, guidelines, chemical incompatibility matrices, and tables presented below are recommended good practices. These are optional guidelines.
- General Recommendations
- Shelves and racks should have enough clearance to accommodate the largest container with room for it to be removed and returned without tipping. Tipping containers when returning them to shelves, cabinets, and refrigerators/freezers may cause the contents to drip or leak.
- Limit hazardous materials kept in fume hoods to the amount that is in use or needed for an activity.
- Avoid stockpiling chemicals.
- Purchase only what is needed. If possible, borrow chemicals from a colleague or contact the Chemical Management System Coordinator (cms@lbl.gov) to assist you in finding a source of the chemical at Berkeley Lab.
- Conduct periodic cleanouts to minimize accumulating unwanted chemicals.
- Chemical Incompatibility Matrices and Tables. Chemical incompatibility data are presented in Tables K-2 and K-3 below. These are recommended guidelines that may be used in combination with container labels, SDSs, and user knowledge for storing and segregating chemicals. An EHS Health and Safety Representative may also be consulted.
- General Recommendations
Table K-1 Refrigerator/Freezer Labeling Requirements:
The Following Labels Shall be Affixed to the Indicated Units
| Unit Type | No Label Required | No Food, beverage or ice1 | Manufacturer’s Flammable Liquid Storage Label2 | No Flammable Storage3 |
| Domestic units in non-technical areas4 | X | NA | NA | NA |
| Domestic units in technical areas5 | NA | X | NA | X |
| Flammable/Combustible Liquid Storage in technical areas6 | NA | X | X | NA |
- See Image K-1.1 below. (Handwritten labels that are legible are allowed.)
- See Image K-1.2 below – this is an example of a label affixed by the manufacturer to a refrigerator approved for storing flammable liquids. There are variations in labels among different manufacturers. No handwritten labels are permitted.
- Fire regulations (NFPA Standard 45) require this label. See Image K-1.3 below. Handwritten labels that are legible are allowed.
- For food storage in areas such as conference rooms, break rooms, kitchenettes and offices.
- For storage of nonflammable material such as media and samples as well as ice, which is not intended for consumption.
- For storage of flammable and combustible liquids. Must be designed and built for that purpose. Refer to Work Process N. (Section 10.d.iv) for additional safety requirements.
| Image K1-1 | Image K1-2 | Image K1-3 |
Table K-2. Incompatibilities by Hazard Class
| Acids, inorganic | Acids, oxidizing | Acids, organic | Alkalis (bases) | Oxidizers | Poisons, inorganic | Poisons, organic | Water- reactives | Organic solvents | |
| Acids, inorganic | X | X | X | X | X | X | |||
| Acids, oxidizing | X | X | X | X | X | X | |||
| Acids, organic | X | X | X | X | X | X | X | ||
| Alkalis (bases) | X | X | X | X | X | X | |||
| Oxidizers | X | X | X | X | |||||
| Poisons, inorganic | X | X | X | X | X | X | |||
| Poisons, organic | X | X | X | X | X | X | |||
| Water-reactives | X | X | X | X | X | X | |||
| Organic solvents | X | X | X | X | X |
X = incompatible
Table K-3. Chemical Incompatibility Table
| Chemical | Keep out of Contact With |
| Acetic acid | Chromic acid, nitric acid, perchloric acid, peroxides, permanganates and other oxidizers |
| Acetone | Concentrated nitric and sulfuric acid mixtures, and strong bases |
| Acetylene | Chlorine, bromine, copper, fluorine, silver, mercury |
| Alkali metals | Water, carbon tetrachloride, or other chlorinated hydrocarbons, carbon dioxide, halogens |
| Ammonia, anhydrous | Mercury, chlorine, calcium hypochlorite, iodine, bromine, hydrofluoric acid |
| Ammonium nitrate | Acids, metal powders, flammable liquids, chlorates, nitrites, sulfur, finely divided organic or combustible materials |
| Aniline | Nitric acid, hydrogen peroxide |
| Arsenic materials | Any reducing agent |
| Azides | Acids |
| Bromine | Same as chlorine |
| Calcium oxide | Water |
| Carbon (activated) | Calcium hypochlorite, all oxidizing agents |
| Carbon tetrachloride | Sodium |
| Chlorates | Ammonium salts, acids, metal powders, sulfur, finely divided organic or combustible materials |
| Chromic acid and chromium trioxide | Acetic acid, naphthalene, camphor, glycerol, glycerin, turpentine, alcohol, flammable liquids in general |
| Chlorine | Ammonia, acetylene, butadiene, butane, methane, propane (or other petroleum gases), hydrogen, sodium carbide, turpentine, benzene, finely divided metals |
| Chlorine dioxide | Ammonia, methane, phosphine, hydrogen sulfide |
| Copper | Acetylene, hydrogen peroxide |
| Cumene hydroperoxide | Acids, organic or inorganic |
| Cyanides | Acids |
| Flammable liquids | Ammonium nitrate, chromic acid, hydrogen peroxide, nitric acid, sodium peroxide, halogens |
| Hydrocarbons | Fluorine, chlorine, bromine, chromic acid, sodium peroxide |
| Hydrocyanic acid | Acids |
| Hydrofluoric acid | Ammonia, aqueous or anhydrous, bases and silica |
| Hydrogen peroxide | Copper, chromium, iron, most metals or their salts, alcohols, acetone, organic materials, aniline, nitromethane, flammable liquids |
| Hydrogen sulfide | Fuming nitric acid, other acids, oxidizing gases, acetylene, ammonia (aqueous or anhydrous), hydrogen |
| Hypochlorites | Acids, activated carbon |
| Iodine | Acetylene, ammonia (aqueous or anhydrous), hydrogen |
| Mercury | Acetylene, fulminic acid, ammonia |
| Nitrates | Sulfuric acid |
| Nitric acid (concentrated) | Acetic acid, aniline, chromic acid, hydrocyanic acid, hydrogen sulfide, flammable liquids, flammable gases, copper, brass, any heavy metals |
| Nitrites | Acids |
| Nitroparaffins | Inorganic bases, amines |
| Oxalic acid | Silver, mercury |
| Oxygen | Oils, grease, hydrogen; flammable liquids, solids, or gases |
| Perchloric acid | Acetic anhydride, bismuth and its alloys, alcohol, paper, wood, grease, and oils |
| Peroxides, organic | Acids (organic or mineral); avoid friction, store cold |
| Phosphorus (white) | Air, oxygen, alkalis, reducing agents |
| Potassium | Carbon tetrachloride, carbon dioxide, water |
| Potassium chlorate and perchlorate | Sulfuric and other acids, alkali metals, magnesium, calcium. |
| Potassium permanganate | Glycerin, ethylene glycol, benzaldehyde, sulfuric acid |
| Selenides | Reducing agents |
| Silver | Acetylene, oxalic acid, tartaric acid, ammonium compounds, fulminic acid |
| Sodium | Carbon tetrachloride, carbon dioxide, water |
| Sodium nitrite | Ammonium nitrate and other ammonium salts |
| Sodium peroxide | Ethyl or methyl alcohol, glacial acetic acid, acetic anhydride, benzaldehyde, carbon disulfide, glycerin, ethylene glycol, ethyl acetate, methyl acetate, furfural |
| Sulfides | Acids |
| Sulfuric acid | Potassium chlorate, potassium perchlorate, potassium permanganate (or compounds with similar light metals, such as sodium, lithium, etc.) |
| Tellurides | Reducing agents |
(From Manufacturing Chemists’ Association, Guide for Safety in the Chemical Laboratory, pp. 215–217, Van Nostrand)
Work Process L. Specific Controls and Procedures — Acids and Bases
- Acids and bases are corrosive and will destroy body tissue. The extent of injury depends on factors such as the type and concentration of the chemical, the route of exposure, the type of tissue contacted, and the speed used in applying emergency measures. Acids, especially in concentrated form, are most likely to cause immediate pain upon contact with tissues. High concentrations of hydrofluoric acid will cause immediate pain and tissue destruction. These effects may be delayed by several hours with weaker concentrations. Fluoride ion from hydrofluoric acid also penetrates the deep tissue layers and can cause bone damage. Skin contact with strong bases usually goes unnoticed, since pain does not occur immediately.
- The eyes are especially susceptible to acids and bases and must be immediately flushed with water for at least 15 minutes if exposure occurs. Inhaling acid fumes and airborne dust and mist from bases irritates the nose, throat, and lungs. Pulmonary edema, a severe irritation of the lungs resulting in fluid production that prevents the transfer of oxygen to the bloodstream, can also occur from intense extreme airborne exposures. Secondary toxic effects may occur if the material is absorbed from the lungs into the bloodstream. The extent of these effects depends on the concentration in air and the duration of exposure. Ingestion causes severe burns of the mucous membranes of the mouth, throat, esophagus, and stomach.
- Dilution of acids and bases is exothermic. This is particularly true for sulfuric acid and potassium hydroxide. Concentrated solutions of inorganic acids and bases are not in themselves flammable. Combustion can occur, however, when an oxidizing acid is mixed with other chemicals or with combustible materials. Acids also react with many metals, resulting in the liberation of hydrogen, a highly flammable gas. Bases such as sodium hydroxide will liberate hydrogen gas upon reaction with aluminum, magnesium, tin, and zinc metal. Some acids are strong oxidizing agents and can react destructively and violently when they come in contact with organic or other oxidizable materials. Perchloric acid may form explosive perchlorate crystals, which are shock-sensitive and can detonate. Acids can form toxic reaction products when combined with cyanide or sulfide salts. The corresponding products are hydrogen cyanide and hydrogen sulfide gas.
Note Regarding Hydrofluoric Acid Generators: Many chemicals containing fluorine atoms, such as ammonium fluoride, sodium fluoride, sulfur tetrafluoride, trifluoracetic acid and ammonium bifluoride, may react with acid or water to produce HF. HF can release hydrogen gas in contact with some metals which could present a flammability hazard. HF can also release toxic gases in contact with various chemicals or when in contact with water. The use of HF generators should be treated with similar precautions as HF and should be identified with their own WPC hazard and controls. - Control Measures
- Activity leads are responsible for identifying acids and bases used in the work area. Review sources such as SDSs for specific compounds.
- An assessment of the hazards and controls in place is necessary to limit employee exposures to these agents. Contact an EHS Health and Safety Representative to provide assistance. This is especially important for hydrofluoric and perchloric acids, aqua regia, and piranha etch.
- Work involving these materials shall be documented by a WPC activity in accordance with the provisions in the Work Planning and Control program (EH&S Manual Chapter 6).
- Training and Information
- Employees who either handle or who may be exposed to the hazards of acids and bases are required to complete Chemical Hygiene and Safety Training (EHS0348; or EHS0345 for Facilities personnel).
Activity leads are responsible for on-the-job training specific to hazards and controls of these materials for their work activities. Information on hazards and minimum PPE requirements must be available to workers accessing work areas where these hazards are present such as through the entrance placard and co-located hazards in WPC activities. EHS Health and Safety Representatives are available to provide assistance.
- Consult Work Process Y, Container Labeling, for labeling requirements for primary and secondary containers.
- The area entrance must be posted with a Caution Placard that depicts hazards and conveys hazard and emergency contact information.
- Employees who work with hydrofluoric acid, hydrofluoric acid generators, or tetramethylammonium hydroxide are required to have additional training. Refer to Work Process W, Training.
- Substitution and Chemical Inventory Management
- Identify and use safer chemical alternatives if possible.
- If a safer chemical can’t be used, limit what you buy or borrow what you need from a colleague in your group or contact the Chemical Management System Coordinator (cms@lbl.gov) for assistance in locating a source of the chemical at Berkeley Lab.
- Conduct periodic cleanouts to prevent accumulation of unnecessary chemicals.
- Procure and use the minimum amount of material required for the operation, or
- Keep working quantities of chemicals to a minimum. Don’t stockpile chemicals.
- Enter these materials into the Chemical Management System (CMS).
- Ventilation
- A fume hood or other appropriate exhaust ventilation must be used when handling acids and bases in a manner that may produce an airborne hazard (such as fumes, gases, vapors, and mists). This includes procedures such as transfer operations, preparation of mixtures, blending, sonication, spraying, and heating.
- Operations involving heating or evaporating perchloric acid must be evaluated by an EHS Health and Safety Representative to determine whether special controls (such as using an acid fume hood with wash-down systems to prevent the accumulation of explosive perchlorate crystals) are needed.
- Work Practices
- Transfer containers of acid and base solutions in bottle carriers.
- Do not pour water into acid. Slowly add the acid to the water and stir.
- Never empty carboys or drums of chemicals by means of air pressure. Use a tilting rack, a safety siphon, or a liquid pump.
- Use a mechanical aid or a pipette bulb for pipetting.
- Open bottles or carboys slowly and carefully, and wear protective equipment to guard hands, face, and body from splashes, vapors, gases, and fumes.
- Wipe drips from containers and bench tops. Be especially careful to wipe up visible residues of sodium hydroxide and potassium hydroxide from all surfaces. Skin contact with dry residue will result in burns.
- Do not eat; drink; smoke; chew gum; apply cosmetics; or store food, beverages, and tobacco products in work areas where acids and bases are being used.
- Personal Protective Equipment (PPE)
- Skin and eye contact must be prevented. The following PPE should be worn when handling these materials. Additional information may be found in Work Process I, Personal Protective Equipment.
- At a minimum, safety glasses with side shields, laboratory coats (coveralls are acceptable in shop settings), and closed-toe shoes will be worn when handling these materials. This is to be considered as minimum protection and must be upgraded if necessary.
- Additional PPE such as chemical goggles, face shields, chemical aprons, disposable coveralls, chemically resistant gloves, and respiratory protection must be worn if there is a greater chance of chemical exposure. An EHS Health and Safety Representative may be contacted for assistance in selecting appropriate gloves and respiratory protection. The use of respiratory protection requires an industrial-hygiene hazard evaluation and a medical clearance followed by a fit test and training by the Research Support Team.
- Consult Work Process I.6, Eye and Face Protection, for guidance on the selection, uses, and limitations of safety glasses, chemical goggles, and face shields.
- The primary concerns with acids and bases are chemical burns. However, since many chemicals are skin-absorbers (i.e., agents that readily pass through the skin), it is important to select gloves that are chemically resistant to the material. Consult Work Process I, Personal Protective Equipment, which contains a list of skin-absorbing agents and provides detailed guidance for selecting chemically resistant gloves.
- Gloves must be selected on the basis of their chemical resistance to the material(s) being handled, their suitability for the procedures being conducted, and their resistance to wear as well as temperature extremes. Improper selection may degrade the gloves, allow the chemical to permeate the gloves and ultimately expose the wearer to the chemical. This is a potentially serious situation. Consult Work Process I.5, Gloves, for guidance on the selection, uses, limitations, and disposal of chemically resistant gloves. An EHS Health and Safety Representative may also be contacted for assistance in selecting appropriate gloves.
- Note Regarding Chemically Resistant Gloves for Hydrofluoric Acid: Although Best 8005 N-Dex Plus nitrile gloves are provided with the HF Exposure Kit (discussed below) and are suitable for applying calcium gluconate gel once the acid has been flushed from the skin, these may not be the appropriate gloves for handling hydrofluoric acid during laboratory operations, as they offer limited chemical resistance under heavy exposure conditions. In selecting the appropriate gloves for hydrofluoric acid, as with all chemicals, the following considerations must be made: the gloves’ chemical resistance to the material(s) being handled, their suitability for the procedures being conducted, and their resistance to wear as well as temperature extremes. Consult Work Process I.5, Gloves, for further information and glove selection. The Web link to the ChemRest Guide to Chemical Resistant Best Gloves provided in that section lists a number of alternatives to the Best 8005 N-Dex Plus nitrile gloves. If using a hydrofluoric acid generator under conditions that generate HF, typically aqueous conditions, have HF gloves on in close proximity.
- Note Regarding PPE for Tetramethylammonium Hydroxide (TMAH) and Solutions, Etchants, and Developers Containing TMAH: TMAH resistant gloves are required. TRIonic tri-polymer gloves sold by MAPA generally provide good protection against concentrated and dilute TMAH solutions. If fine dexterity is important, double gloving with nitrile gloves may be used only for < 3% TMAH solutions if there is no gap between the lab coat and gloves by using arms sleeves or longer nitrile gloves (12”). Change the outer glove often and immediately if it becomes wetted. A TMAH-resistant apron such as Silver Shield/4H Apron is required when handling large quantities of TMAH or when there is a splash potential. A face shield (in addition to safety glasses with side shields) is required when handling 3% or greater TMAH solutions or when there is a splash potential for work involving any concentration of TMAH. Refer to the TMAH Best Practices Guide for more information and PPE resources.
- Storage
- Consult Work Process K, Chemical Storage, for hazardous material storage requirements, recommendations, and information on chemical incompatibility. Additional requirements are provided below.
- Segregate acids from bases.
- Segregate acids from reactive metals such as sodium, potassium, and magnesium.
- Segregate oxidizing acids from organic acids and flammable and combustible materials.
- Segregate acids from chemicals that could generate toxic or flammable gases upon contact, such as sodium cyanide, iron sulfide, and calcium carbide.
- Store inorganic acids in corrosive- or acid-storage cabinets. Their interiors and hardware (door hinges and shelf brackets) are corrosion resistant. Corrosive-storage cabinets can be located under fume hoods or exist as stand-alone units. Flammable-storage cabinets are not corrosion resistant and must not be used for acid storage.
- Store acids and bases in sealed, air-impermeable containers with tight-fitting caps, as opposed to loose-fitting lids or glass stoppers. An exception to this is mixtures that may produce gases that can pressurize the container. These include piranha etch and aqua regia. Piranha etch is a mixture of 98% sulfuric acid and 30% hydrogen peroxide in ratios ranging from 2-4:1. It produces gaseous oxygen. Aqua regia is a 1:3 mixture of concentrated nitric and hydrochloric acids. It produces nitrogen dioxide, chlorine, and nitrosyl chloride gases. Either mix fresh batches and use on the same day, or fit containers with vented caps to prevent over-pressurization.
- Keep piranha etch and aqua regia in fume hoods at all times. Note: Normally hazardous materials kept in fume hoods should be limited to those that are in use or that are needed for an activity. But because piranha etch and aqua regia may off-gas, these should be kept in a fume hood.
- Do not store aqueous sodium and potassium hydroxide solutions in aluminum drip trays. These will corrode aluminum and compromise its integrity.
- Store nitric acid in its own secondary containment trays. Nitric acid can combine with other acids to form nitrogen oxides and nitrosyl halide gases.
- Combustible organic carboxylic acids such as formic and acetic acids may be stored in a flammable storage cabinet along with other flammable and combustible liquids. These acids do not pose the same corrosive and oxidizing hazards of other mineral and oxidizing acids.
- Emergency Procedures
- Consult Work Process V, Emergency Procedures and Equipment, for emergency actions regarding chemical spill and personal exposure to chemicals.
- In addition to these requirements, the following applies to acid and base spills:
- Never use combustible or reactive materials (such as paper towels) to clean up or absorb spills. Keep an adequate number of appropriate spill kits to meet anticipated needs.
- Do not clean up or neutralize acid spills with bases (including soda ash, sodium carbonate). In addition, do not neutralize base spills with acids. A potential aggressive and exothermic reaction may ensue. Gaseous carbon dioxide generated from the neutralization reaction can cause splattering.
- Use commercially available acid and base spill cleanup kits that contain “neutralizing agents” and acid/base (pH) indicators. These neutralize spills at a controlled reaction rate, which eliminates splattering and excessive heat generation. The pH indicator provides a visible color change to indicate complete neutralization of the spill. These are available through VWR Scientific.
- It is important to have a sufficient quantity of neutralizer to handle anticipated needs. For example, a J.T. Baker spill kit contains 3.2 kg of neutralizing agent. This is sufficient to neutralize the volumes of the acids indicated in Tables L-1 and L-2.
- Ensure that you read and understand how to use spill cleanup and neutralizing agents before a spill occurs.
- Add neutralizing agents slowly and deliberately. Understand that a chemical reaction will occur that involves some heat generation and the evolution of gas (normally carbon dioxide).
- Note Regarding Spill Kits for Hydrofluoric Acid or HF Generators: Hydrofluoric acid cannot be cleaned up with normal acid spill kits. Do not use silica-containing agents, especially diatomaceous earth (kitty litter) and sand, to absorb hydrofluoric acid, because they can react with HF to form toxic silicon tetrafluoride gas. Use spill kits such as HF Acid Eater (NPS Corp.) or HF Spill Tamer (J.T. Baker/Mallinckrodt). HF is a weak acid and does not completely dissociate. Therefore, sufficient time must be allowed for the neutralizing agent to neutralize the acid.
- An emergency eyewash and safety shower must be located in all areas where acids or bases are used. In the event of skin or eye contact, flush the affected area for at least 15 minutes and report to Health Services for evaluation and treatment.
- Note Regarding Contact with Hydrofluoric Acid and First Aid: Any suspected skin contact with hydrofluoric acid, HF generator, or gas should be treated with flushing as described above, except that flushing should be done for a 5-minute instead of a 15-minute period. Flushing may remove surface hydrofluoric acid, but it does not affect the fluoride ion, which may have penetrated to the deep tissue layers. All work areas where hydrofluoric acid is used must have at least one HF exposure kit. These consist of calcium gluconate gel, Best 8005 N-Dex Plus nitrile gloves for applying the gel, and instructions on what to do in case of exposure. (See Note Regarding Chemically Resistant Gloves for Hydrofluoric Acid, above, regarding the limitations of Best 8005 N-Dex Plus nitrile gloves for normal laboratory operations). Hydrofluoric acid exposure kits are available through Health Services (ext. 6266). Calcium Gluconate must be acquired through Health Services as medical supplies are a restricted purchasing item. If exposed, flush the affected area for 5 minutes, don the Best 8005 N-Dex Plus (8-mil-thick) nitrile gloves, liberally apply the gel to the affected areas (not the eyes), and report to Health Services immediately. This is effective from 7:30 A.M. to 4:30 P.M. After hours, emergency medical services are available from the Fire Department through the 7-911 dispatch system.
- Note Regarding Contact with TMAH solutions: Skin exposure to >1% TMAH over a few percent of the body must be treated as a life-threatening event. Designate someone to call 911 and activate the safety shower immediately. SDSs may be out of date and may not properly describe this high dermal toxicity. Do not work alone with 25% or greater TMAH. Avoid working alone with more dilute solutions. Prepare for “Off normal” events such as a spill, splash, or other emergency.
Tables for Work Process L
Table L-1
| Acid and Concentration | Volume (L) Acid Neutralized by 3.2 kg of JT Baker Neutrasorb® |
| Hydrobromic acid (38%) | 2.6 |
| Hydrochloric (38%) | 1.9 |
| Hydroiodic acid (51%) | 6.3 |
| Nitric acid (71%) | 1.5 |
| Perchloric acid (72%) | 2.0 |
| Phosphoric (87%) | 0.5 |
| Sulfuric acid (98%) | 0.7 |
| Sulfurous acid (9%) | 11.5 |
The J.T. Baker Neutracit® caustic spill cleanup kit has 1.2 kg of neutralizing agent, which has the following neutralizing capacities:
Table L-2
Base and Concentration | Volume (L) Base Neutralized by 1.2 kg of JT Baker Neutracit® |
| Ammonium hydroxide (28%) | 0.75 |
| Potassium hydroxide (45%) | 0.66 |
| Sodium hydroxide (50%) | 0.47 |
Work Process M. Specific Controls and Procedures — Particularly Hazardous Substances: Carcinogens, Reproductive Toxins, and Acute Toxins
Cal/OSHA has established a category of chemicals known as particularly hazardous substances for which special precautions may be required. Particularly hazardous substances include select carcinogens, reproductive toxins, and substances with a high degree of acute toxicity. The Web resources listed below, container labels, and SDSs should be reviewed to identify these hazards. The Chemical Management System (CMS) can also be used to identify these substances in a work area.
- Carcinogens. Carcinogens are agents that cause neoplasms (tumors) in humans and/or animals. Carcinogenic agents may be organic chemicals, inorganic chemicals, or hormones. Some carcinogens react directly with a cell’s genetic information (the DNA), causing changes (mutations) that are incorporated into subsequent generations of that cell. Select carcinogens are agents that are strongly implicated as sources of cancer in humans. In addition, Cal/OSHA has established research laboratory use requirements for 13 regulated carcinogens. Regulated carcinogens are included on the restricted items list and require EHS approval prior to purchasing. Lists of select carcinogens and other resources are available in Appendix C of this program.
- Reproductive Toxins. Reproductive toxins are chemicals that can damage the reproductive systems of both men and women. They can affect reproductive capabilities, including chromosomal damage (mutations), and produce effects on developing fetuses (teratogenesis). Reproductive toxins can affect both men and women. Examples of adverse reproductive health effects include birth defects, spontaneous abortion, fetal developmental damage, and infertility. It is important to note that the first trimester of pregnancy is the period of most concern to the developing fetus because this is when the organs and the limbs are being formed. During this period, many women may not yet be aware that they are pregnant. For this reason, it is important that the use of reproductive toxins have been identified and that control measures are in place to protect a woman and her fetus from harmful exposure levels. Women who are (or are trying to become) pregnant may consult with Health Services before the start of any laboratory or shop activity involving reproductive toxins. A list of reproductive toxins that affect women as well as men is provided in Appendix D.
- Acutely Toxic Substances. Substances of high acute toxicity include materials that may be fatal or cause damage to target organs from a single exposure or from exposures of short duration. They also include materials capable of causing intense irritation that can result in pulmonary edema (fluid and swelling in the lungs), chemical asphyxia, and systemic (body-wide) poisoning. It is not practical to provide a list of all substances of high acute toxicity in this document. The SDS should be consulted to determine the toxicity of all substances. An EHS Health and Safety Representative may also be consulted for additional guidance.
- Control Measures
- Activity leads are responsible for identifying particularly hazardous substances used in the work area. Review sources such as SDSs for specific compounds.
- An assessment of the hazards and controls in place is necessary to limit employee exposures to these agents. Contact an EHS Health and Safety Representative to provide assistance.
- Work involving these materials shall be documented by a WPC activity in accordance with the provisions in the Work Planning and Control program (EH&S Manual Chapter 6).
- Cal/OSHA requires that the following four categories of controls be considered for operations and activities involving particularly hazardous substances:
- Establish posted designated areas. A designated area may be a room, a section of a room, a bench top or a containment device (such as a lab hood). Requirements are found in Work Process BB, Designated Areas. NOTE: When handling substances (in non-laboratory settings) that are regulated by Cal/OSHA substance-specific standards (such as asbestos), “regulated areas” will be established in accordance with the applicable Cal/OSHA standard.
- Implement contaminated-waste removal procedures. Contaminated wastes shall be collected in impervious containers, which are closed and decontaminated prior to removal from the work area. Refer to Berkeley Lab waste-handling policy and procedures as addressed in the ES&H Manual Waste Management program for additional requirements.
- Use containment devices (such as fume hoods, gas cabinets, glove boxes or the equivalent).
- Implement contaminated-waste removal procedures. NOTE: Compliance with Berkeley Lab waste-handling policy and procedures as addressed in the ES&H Manual Waste Management program satisfies this requirement.
- Establish decontamination procedures. These are necessary to prevent the spread of contamination to other areas. Decontamination procedures include practicing good housekeeping by wiping down work surfaces at the end of the day and cleaning up drips, residues, and spills. Cleanup materials used (such as absorbents and cloths) must be disposed of as hazardous waste. NOTE: Wipe sampling, as described in Work Process CC, Exposure Assessment, Monitoring, and Medical Consultation, may be required to confirm the effectiveness of decontamination procedures.
- Training and Information
- Employees who either handle or who may be exposed to particularly hazardous substances must complete Chemical Hygiene and Safety Training (EHS0348; or 345 for Facilities personnel).
Activity leads are responsible for on-the-job training specific to hazards and controls of these materials for their work activities. Furthermore, employees working in designated areas are to be informed of the specific hazards and controls of the materials used. Information on hazards and minimum PPE requirements must be available to workers accessing work areas where these hazards are present such as through the entrance placard and co-located hazards in WPC activities.
EHS Health and Safety Representatives are available to provide assistance.
- Consult Work Process Y, Container Labeling, for labeling requirements for primary and secondary containers.
- The area entrance/designated and regulated areas must be posted with a Caution Placard depicting hazards and emergency contact information.
- Substitution and Chemical Inventory Management
- Identify and use safer chemical alternatives if possible.
- If a safer chemical can’t be used, limit what you buy or borrow what you need from a colleague in your group or contact the Chemical Management System Coordinator (cms@lbl.gov) to assist you in finding a source of the chemical at Berkeley Lab.
- Conduct periodic cleanouts to prevent accumulating unneeded chemicals.
- Procure and use the minimum amount of material required for the operation, or
- Keep working quantities of chemicals to a minimum. Don’t stockpile chemicals.
- Enter these materials into the Chemical Management System (CMS).
- Ventilation
Use local exhaust ventilation such as a fume hood or glove box when handling particularly hazardous substances in a manner that may produce an airborne hazard (such as fumes, gases, vapors, and mists). This includes operations such as transfer operations, preparation of mixtures, blending, sonification, spraying, heating, and distilling. See Work Process H, Selection and Use of Engineering Controls, for more information.
- Work Practices
- Transfer containers in bottle carriers.
- Do not eat, drink, smoke, chew gum or tobacco, store food, or apply cosmetics in work or storage areas.Use a mechanical aid or a pipette bulb for pipetting.
- Use a mechanical aid or a pipette bulb for pipetting.
- Open bottles or carboys slowly and carefully and wear protective equipment to guard hands, face, and body from splashes and vapors/gases.
- Wipe drips/residues from containers and work surfaces. To facilitate decontamination, use stainless steel or plastic trays, absorbent paper with a moisture-proof lining, or other impervious material.
- Upon completion of the operation, decontaminate or discard the protective covering material as hazardous waste.
- Wash hands before leaving the work area and prior to consuming food/beverages.
- Do not eat; drink; smoke; chew gum; apply cosmetics; or store food, beverages, and tobacco products in work areas where particularly hazardous substances are being used.
- Personal Protective Equipment (PPE). Skin and eye contact must be prevented. The following PPE should be worn when handling these materials. Additional information may be found in Work Process I, Personal Protective Equipment.
- At a minimum, safety glasses with side shields, laboratory coats (coveralls are acceptable in shop settings), and closed-toe shoes will be worn when handling these materials. This is to be considered as minimum protection and must be upgraded if necessary.
- Additional PPE such as chemical goggles, face shields, chemical aprons, disposable coveralls, chemically resistant gloves, and respiratory protection must be worn if there is a greater chance of chemical exposure. An EHS Health and Safety Representative may be contacted for assistance in selecting appropriate gloves and respiratory protection. The use of respiratory protection requires an industrial hygiene hazard evaluation and a medical clearance followed by a fit test and training by the Research Support Team.
- Consult Work Process I.6, Eye and Face Protection, for guidance on the selection, uses, and limitations of safety glasses, chemical goggles, and face shields.
- Because many chemicals are skin-absorbers (i.e., agents that readily pass through the skin) it is important to select gloves that are chemically resistant to the material. Consult the PPE section, which contains a list of skin-absorbing agents and provides detailed guidance for selecting chemically resistant gloves. An EHS Health and Safety Representative may also be contacted for assistance in selecting appropriate gloves.
- Gloves must be selected on the basis of their chemical resistance to the material(s) being handled, their suitability for the procedures being conducted, and their resistance to wear as well as temperature extremes. Improper selection may result in glove degradation, permeation of the chemical through the glove, and ultimately, personal exposure to the chemical. This is a potentially serious situation. Consult Work Process I.5, Gloves, for guidance on the selection, uses, limitations, and disposal of chemically resistant gloves. An EHS Health and Safety Representative may also be contacted for assistance in selecting appropriate gloves.
- Storage
- Consult Work Process K, Chemical Storage, for hazardous-material storage requirements, recommendations, and information on chemical incompatibility. Additional requirements are provided below.
- Follow the storage guidelines in Work Process N, Specific Controls and Procedures — Flammables and Combustible Liquids, if the material is either flammable or combustible.
- Emergency Procedures. Refer to Work Process V, Emergency Procedures and Equipment, for Berkeley Lab policy and response procedures for chemical spills and personal exposure to chemicals.
Work Process N. Specific Controls and Procedures — Flammables and Combustible Liquids
- General Information
- Flammable and combustible chemicals include liquids such as organic solvents, oils, greases, tars, oil-based paints, and lacquers, as well as flammable gases. Flammable gases are discussed in ESH Manual programs Chapter 7, Pressure Safety, and Chapter 13, Gas Safety. The emphasis of this section is on flammable and combustible liquids.
- Flammable and combustible liquids are defined by their flashpoints. The flashpoint of a liquid is the minimum temperature at which it gives off sufficient vapor to form an ignitable mixture with the air near its surface or within its containment vessel. A liquid’s flashpoint is a function of its vapor pressure and boiling point. Generally, the higher the vapor pressure and the lower the boiling point of a liquid, the lower its flashpoint will be. The lower the flashpoint, the greater the fire and explosion hazard.
- Flammable and combustible liquids are classified by the National Fire Protection Association (NFPA) and the California Fire Code (CFC) based on their flashpoints.
- Flammable Liquids (Class I)
- Liquids with flashpoints below 100°F (37.8°C) and vapor pressures not exceeding 40 pounds per square inch (absolute) at 100°F (37.8°C). The boiling point is only considered when distinguishing between Class IA and IB flammables. Flammable Class I liquids are subdivided as follows:
| Class | Flashpoint | Boiling Point | Examples |
| IA | <73°F (<22.8°C) | <100°F (<37.8°C) | Diethyl ether, n-pentane, acetaldehyde |
| IB | <73°F (<22.8°C) | >100°F (>37.8°C) | Acetone, ethanol, isopropanol, methanol, toluene, tetrahydrofuran, gasoline |
| IC | >73°F to <100°F(>22.8°C to <37.8°C) | Not considered | 1-pentanol, xylene, naphtha, ethylenediamine, butanol |
- Combustible Liquids (Classes II and III)
- Liquids having flashpoints at or above 100°F (37.8°C). The boiling point is not considered. Combustible liquids in Classes II and III are subdivided as follows:
| Class | Flashpoint | Examples |
| II | >100°F to <140°F (>37.8°C to <60.0°C) | Formaldehyde, mineral spirits, glacial acetic acid, formic acid, kerosene |
| IIIA | >140°F to <200°F (>60.0°C to <93.4°C) | Benzaldehyde, aniline, ethanolamine, dimethylsulfoxide, phenol |
| IIIB | >200°F (>93.4°C) | Triethanolamine, ethylene glycol, propylene glycol, mineral oil, oleic acid |
- Control Measures
- Work leads must identify flammable and combustible liquids used in the work area. Review sources such as SDSs for specific compounds.
- An assessment of the hazards and controls in place is necessary to limit employee exposures to these agents. Contact an EHS Health and Safety Representative to provide assistance.
- Work involving these materials shall be added to a Work Planning and Control Activity. Consult the Work Planning and Control program (EHS Manual Chapter 6).
- Training and Information
- Employees who either handle or who may be exposed to flammable and combustible liquids are required to complete Chemical Hygiene and Safety Training (EHS 0348; or EHS 0345 for Facilities personnel).
- Activity leads are responsible for on-the-job training specific to hazards and controls of these materials for their work activities. Information on hazards and minimum PPE requirements must be available to workers accessing work areas where these hazards are present such as through the entrance placard and co-located hazards in WPC activities. EHS Health and Safety Representatives are available to provide assistance.
- Consult Work Process Y, Container Labeling, for labeling requirements for primary and secondary containers.
- The entrance to the work area should be posted with a Caution Placard depicting hazards and emergency contact information.
- Substitution and Chemical Inventory Management
- Identify and use safer chemical alternatives (e.g., materials with higher flashpoints and higher boiling points) if possible.
- If a safer chemical can’t be used, limit what you buy or borrow what you need from a colleague in your group or contact the Chemical Management System Coordinator (cms@lbl.gov) to assist you in finding a source of the chemical at Berkeley Lab.
- Conduct periodic cleanouts to prevent accumulating unneeded chemicals.
- Keep working quantities of chemicals to a minimum. Don’t stockpile chemicals.
- Enter these materials into the Chemical Management System (CMS).
- Ventilation. A fume hood or other appropriate exhaust ventilation system should be used when handling flammable and combustible liquids in a manner that may produce an airborne hazard (such as fumes, gases, vapors, and mists). This includes procedures such as transfer operations, preparation of mixtures, blending, sonification, spraying, heating, and distilling. Consult Work Process N.11, “Transfer Operations,” for guidance and requirements regarding ventilation during transfers from one container to another.
- Work Practices
- Control all ignition sources when handling flammable and combustible liquids. Sources of ignition include open flames, smoking, hot surfaces, electrical and mechanical sparks, cutting and welding, static electricity, heat-producing chemical reactions, and anything that closes an electrical circuit (e.g., opening/closing a switch, plugging/unplugging a power cord, electrical motor or compressor switching on/off, etc.). Contact your EHS Health and Safety Representative or the Fire Marshal for assistance in identifying ignition sources.
- Consult Work Process N.11, “Transfer Operations,” for guidance and requirements on transferring flammable and combustible liquids from one container to another, including bonding and grounding information.
- Keep containers closed when not in use.
- Storing and consumption of food is permitted in designated areas only. See Work Process J, Work Practice Controls for additional information.
- Use a mechanical aid or a pipette bulb for pipetting.
- Open bottles or carboys slowly and carefully and wear protective equipment to guard hands, face, and body from splashes and vapors/gases.
- Wipe drips/residues from containers and work surfaces.
- Wash hands before leaving the work area and prior to consuming food/beverages.
- Personal Protective Equipment (PPE). Skin and eye contact must be prevented. The following PPE should be worn when handling these materials. Additional information may be found in Work Process I, Personal Protective Equipment.
- At a minimum, safety glasses with side shields, laboratory coats (coveralls are acceptable in shop settings) and closed-toe shoes will be worn when handling these materials. This is to be considered as minimum protection and must be upgraded if necessary.
- Additional PPE such as chemical goggles, face shields, chemical aprons, disposable coveralls, chemically resistant gloves and respiratory protection must be worn if there is a greater chance of chemical exposure. An EHS Health and Safety Representative may be contacted for assistance in selecting appropriate gloves and respiratory protection. The use of respiratory protection requires an industrial-hygiene hazard evaluation and a medical clearance followed by a fit test and training by the Industrial Hygiene Group.
- Consult Work Process I.6, Eye and Face Protection, for guidance on the selection, uses, and limitations of safety glasses, chemical goggles, and face shields.
- Because many chemicals are skin-absorbers (i.e., agents that readily pass through the skin) it is important to select gloves that are chemically resistant to the material. Consult the PPE section. This contains a list of skin-absorbing agents and provides detailed guidance for selecting chemically resistant gloves.
- Gloves must be selected on the basis of their chemical resistance to the material(s) being handled, their suitability for the procedures being conducted, and their resistance to wear as well as temperature extremes. Improper selection may result in glove degradation, permeation of the chemical through the glove, and ultimately personal exposure to the chemical. This is a potentially serious situation. Consult Work Process Work Process I.5, Gloves, for guidance on the selection, uses, limitations, and disposal of chemically resistant gloves. An EHS Health and Safety Representative may also be contacted for assistance in selecting appropriate gloves.
- Storage
- Consult Work Process K, Chemical Storage, for hazardous-materials storage requirements, recommendations, and information on chemical incompatibility. Additional requirements are provided below.
- Store flammable and combustible liquids away from ignition sources. Sources of ignition include open flames, smoking, hot surfaces, electrical and mechanical sparks, cutting and welding, static electricity, heat-producing chemical reactions, and anything that closes an electrical circuit (e.g., opening/closing a switch, plugging/unplugging a power cord, electrical motor or compressor switching on/off, etc.). Contact your EHS Health and Safety Representative for assistance in identifying ignition sources.
- Segregate flammable and combustible liquids from oxidizing acids and oxidizers.
- Flammable-Storage Cabinets and Refrigerators/Freezers.
- Store flammable and combustible liquids in an approved flammable-storage cabinet.
- Containers of flammable liquids (e.g., squeeze bottles or similar volume secondary containers with closed lids or tops) may be maintained outside of flammable liquid storage cabinets while in use or when staged for use. Staging requires the use of secondary containment as specified in the Chemical Hygiene and Safety Plan (Work Process K, Chemical Storage).
- Unless in use or staged for use, store flammable liquid containers in flammable liquid storage cabinets. Place all flammable liquid containers in flammable liquid storage cabinets when no longer needed.
- Limit the amount of flammable liquids staged for use to the smallest practical quantity.
- Note: Storage of nonflammable solvents such as chloroform and methylene chloride are permitted in flammable storage cabinets provided that (1) they are chemically compatible with the other stored chemicals and (2) storage of non-flammables does not displace flammable and combustible chemicals from the storage cabinet.
- Flammable-storage cabinets are designed to protect their contents from fires in the work area. They can be located under fume hoods or exist as stand-alone units. Approved flammable-storage cabinets are constructed of steel and are equipped with self-closing doors with a three-point latch arrangement. Flammable-storage cabinets installed as part of laboratory construction and renovation projects must be connected to the building’s supply and exhaust ventilation system. The top bung is connected to an outside source of supply air and the lower bung is connected to the exhaust system. This controls vapors and odors and prevents corrosion to the interior.
- No more than 120 gallons of Class I, Class II, and Class IIIA liquids, combined, may be stored in a flammable-storage cabinet. Of this total, no more than 60 gallons may be Class I and Class II liquids, combined. Refer to ES&H Manual Chapter 12, Fire Prevention and Protection, for detailed guidance on maximum quantities allowed in a single fire-control area (i.e., an area that is separated from other rooms/areas by minimum one-hour fire-rated barriers).
- Do not store Class I liquids in any basement or pit unless it has an approved ventilation system designed to prevent the accumulation of flammable vapors (refer to OSHA 1910.106[f][2][iii][b]). A basement is a story of a building or structure having one-half or more of its height below ground level. For questions or further guidance, consult the Berkeley Lab’s Fire Marshal.
- Ordinary domestic refrigerators/freezers contain electrical components (light bulbs, switches, contacts, and motors) that are potential ignition sources that may initiate a fire or an explosion if flammable vapors are present. Therefore, refrigerators/freezers used for storing flammable liquids must be designed, constructed, approved for that purpose. NOTE: This applies to aqueous ethanol solutions greater than or equal to 15%. Domestic refrigerator/freezers as well as units that have been modified to remove spark sources are not acceptable alternatives. Contact Procurement & Property Management for guidance on purchasing refrigerators and freezers.
- Labeling: Refrigerators/freezers approved for storage of flammable materials must be labeled by the manufacturer to indicate this approval and any associated prohibitions, e.g., no smoking, keep flame or fire away, etc. In addition, they must be labeled to indicate no storage of food, beverages, or ice for human consumption. See Work Process K, Table K-1 Refrigerator/Freezer Labeling Requirements.
- Store flammable and combustible liquids in an approved flammable-storage cabinet.
- Flammable and Combustible Storage Cans and Other Containers. Flammable and combustible liquids may be stored in various containers. The allowed volume depends on the flammable/combustible class and container material (See Table N-1 below).
Table N-1. Maximum Container Sizes for Combustible and Flammable Fluids
| Flammable Liquid Class | Combustible Liquid Class | ||||
| Container Type | 1A | 1B | 1C | II | III |
| Glass | 1 pinta(0.5 L)d | 1 quarta(1 L) | 1 gal(4 L) | 1 gal(4 L) | 5.3 gal(20 L) |
| Metal (other than Department of Transportation [DOT] drums) | 1 gal(4 L) | 5.3 gal(20 L) | 5.3 gal(20 L) | 5.3 gal(20 L) | 5.3 gal(20 L) |
| Approved safety cansb | 2.6 gal(10 L) | 5.3 gal(20 L) | 5.3 gal(20 L) | 5.3 gal(20 L) | 5.3 gal(20 L) |
| The below container sizes are not suitable for laboratory operations. Contact EHS for assistance. | |||||
| Metal drums (DOT specifications) | 119 gal(450 L) | 119 gal(450 L) | 119 gal(450 L) | 119 gal(450 L) | 119 gal(450 L) |
| Approved metal portable tanks | 793 gal(3,000 L) | 793 gal(3,000 L) | 793 gal(3,000 L) | 793 gal(3,000 L) | 793 gal(3,000 L) |
a Glass containers of no more than 1 gallon capacity may be used for Class IA or IB flammable liquids if such liquid either would be rendered unfit for its intended use by contact with metal or would excessively corrode a metal container so as to create leakage hazard. NOTE: This exemption does not apply to the accumulation of non-corrosive ignitable hazardous waste.
b Underwriter Laboratory or Factory Mutual approved container equipped with a self-closing lid, pressure relief, flame arrester, bonding/grounding tab, and a funnel.
Note: Class I liquids are flammable, and Class II liquids are combustible; Class 1A liquids have a flashpoint (FP) below 73°F, and boiling point (BP) below 100°F; Class 1B — FP below 73°F, and BP at or above 100°F; Class 1C — FP at or above 73°F, but less than 100°F (BP not addressed); Class II — FP at or above 100°F, but below 140°F; Class III — FP at or above 140°F.
- Transfer Operations
- All Classes of Flammable and Combustible Liquids
- Keep ignition sources at least 3 feet away.
- Bonding and grounding of conductive containers is required when the size of the source container is greater than 1 gallon or 4 liters. Bonding/grounding is recommended whenever a metal or conductive plastic container is involved. Click here for additional guidance and information on bonding and grounding.
- Use secondary containment and have appropriate spill control supplies available.
- Keep containers closed when not in use.
- Class IA Flammable Liquids
- All transfers of Class IA flammable liquids must be performed inside a fume hood or with appropriate local exhaust ventilation.
- Class IA flammable liquids must never be transferred by gravity-dispensing (see 11.c. below).
- Class IB and IC Flammable Liquids
- Transfers of Class IB and IC flammable liquids must be performed inside a fume hood or with appropriate local exhaust ventilation.
Exception: A maximum of 2 liters of Class IB or IC flammable liquids may be transferred within a one-hour period outside of a fume hood or without local exhaust ventilation. Such transfers must take place with adequate room ventilation. Spaces that are enclosed or constricted must be avoided. Contact your EHS Health and Safety Representative for an exposure assessment if there are any concerns about the health hazard of the chemicals being handled. Refer to Work Process CC “Exposure Assessments, Monitoring, and Medical Consultation” for more information.
Note: This exception is based on a study performed by EHS in December 2016 in response to findings from an NFPA 45 inspection. Contact EHS for a copy of the report “Berkeley Lab Revised Assessment of Flammable Vapors and the Need for Local Exhaust Ventilation per NFPA 45 Requirements in Chapters 7.2.6 and 9.3.1” (CHESS ID# SIH-6352).
- Transfers of Class IB and IC flammable liquids must be performed inside a fume hood or with appropriate local exhaust ventilation.
- Class II and III Combustible Liquids
- Transfers of Class II and III combustible liquids – when heated at or above the flashpoint during the transfer – must be performed according to the requirements listed for Class IB and IC flammable liquids above.
- Transfers of unheated Class II and III combustible liquids must be performed in well-ventilated areas. A fume hood or local exhaust ventilation is required if there is a potential that the transfer may produce an airborne physical or health hazard. Contact your EHS Health and Safety Representative for assistance in determining potential for such airborne hazards.
- Gravity-Dispensing Flammable IB and IC and Combustible Liquids.
- Gravity-dispensing refers to transferring flammable or combustible liquids by means of gravity from large containers placed on the side or containers with a valve near the bottom. Container size is usually greater than 20 gallons (75 liters), but can be smaller.
- Class IB, Class IC, Class II, and Class III liquids may be transferred from containers or tanks by gravity through piping, hoses, and self- or automatic closing valves that have been reviewed and approved by Berkeley Lab’s Fire Marshal.
- Such transfer operations must follow the general requirements as outlined in section 11.a above.
- Moreover, the nozzle and conductive containers must be bonded to each other (i.e., electrically interconnected) to prevent static electricity discharge. The dispensing container (if conductive) must also be connected to an electrical ground.
- Ventilation must be provided as outlined in sections 11.c and 11.d above.
- Contact your EHS Health and Safety Representative and the Fire Marshal’s Office for assistance in setting up gravity-dispensing operations.
- All Classes of Flammable and Combustible Liquids
- Emergency Procedures
- Consult Work Process V, Emergency Procedures and Equipment, for emergency actions regarding chemical spill and personal exposure to chemicals.
- In addition to these requirements, the following applies to flammable and combustible liquid spills:
- Never use combustible or reactive materials (such as paper towels) to clean up or absorb spills of flammable or combustible liquids. Keep an adequate number of appropriate spill kits to meet anticipated needs. These are commercially available through VWR Scientific. Typically, products containing diatomaceous earth are used for absorbing organic solvents.
- An emergency eyewash and safety shower should be located in all areas where flammable or combustible liquids are used. In the event of skin or eye contact, flush the affected area for at least 15 minutes and report to Health Services for evaluation and treatment.
Work Process O. Specific Controls and Procedures — Laser Dyes and Solvents
- General
- Dye lasers normally use a lasing medium consisting of a fluorescent organic dye dissolved in an organic solvent. For most dyes, little is known about their toxic properties, except that they are often members of chemical families that contain highly toxic materials. Furthermore, limited testing has indicated that some laser dyes are carcinogenic or mutagenic. Consequently, most dyes should be treated as hazardous chemicals. In many cases, the solvent in which the dye is dissolved plays a major role in the hazards. Most solvents used for dye solutions are flammable and toxic by inhalation and/or skin-absorption.
- The following measures were developed to combine the need for a cautious approach to preventing exposures to hazardous chemicals, proper waste management, fire prevention, and practical operating requirements.
- Control Measures
- Activity leads are responsible for identifying laser dyes and solvents used in the work area. Review sources such as SDSs for specific compounds.
- An assessment of the hazards and controls in place is necessary to limit employee exposures to these agents. Contact an EHS Health and Safety Representative to provide assistance.
- Work involving these materials shall be added to a Work Planning and Control Activity. Consult the Work Planning and Control program (EH&S Manual Chapter 6).
- Training and Information
- Employees who either handle or who may be exposed to laser dyes and solvents must complete Chemical Hygiene and Safety Training (EHS0348; or 345 for Facilities personnel).
- Activity leads are responsible for on-the-job training specific to hazards and controls of these materials for their work activities. Information on hazards and minimum PPE requirements must be available to workers accessing work areas where these hazards are present such as through the entrance placard and co-located hazards in WPC activities. EHS Health and Safety Representatives are available to provide assistance.
- Consult Work Process Y, Container Labeling, for labeling requirements for primary and secondary containers.
- The entrance to the work area must be posted with a Caution Placard depicting hazards and emergency contact information.
- Substitution and Chemical Inventory Management
- Identify and use safer chemical alternatives (e.g., non-mutagenic/carcinogenic dyes or less concentrated forms) if possible. Note that some solvents such as dimethyl sulfoxide (DMSO) and methyl alcohol readily penetrate unbroken skin. Hazardous mutagenic or carcinogenic dyes can enter the body through skin-absorption when dissolved in solvents such as these.
- If a safer chemical can’t be used, limit what you buy or borrow what you need from a colleague in your group or contact the Chemical Management System Coordinator (cms@lbl.gov) to assist you in finding a source of the chemical at Berkeley Lab.
- Conduct periodic cleanouts to prevent accumulating unneeded chemicals.
- Procure and use the minimum amount of material required for the operation, or
- Keep working quantities of chemicals to a minimum. Don’t stockpile chemicals.
- Enter these materials into the Chemical Management System (CMS).
- Engineering Controls
- A fume hood must be used when mixing laser dyes or when handling them in a manner that may generate an airborne hazard (such as fumes, gases, vapors, and mists).
- Install spill pans under pumps and reservoirs or, preferably, enclose them. Make sure that knobs and other protuberances extend through the holes in the enclosures.
- Leak-test dye pump loops, as appropriate.
- Work Practices
- Do not eat; drink; smoke; chew gum; apply cosmetics; or store food, beverages, and tobacco products in work areas where laser dyes and solvents are being used.
- Use mechanical pipetting aids when handling dye solutions.
- Keep containers of solvents and dye solutions closed.
- Cap off and/or drain dye lines that are not in use.
- Keep the work area clean. Use wet methods for housekeeping in dye work areas. Remove visible stains as much as practical during cleanup. (NOTE: Custodians should not do dye cleanup work.)
- Keep flammable solvents in approved storage cabinets.
- Wash hands after handling laser dyes and solutions.
- Personnel who have had skin, eye, or inhalation exposure to dye powders or solutions should contact an EHS Health and Safety Representative.
- Minimize the quantity of pure dye or solutions containing >0.1% of mutagenic/carcinogenic dyes in storage or in use at any time.
- Ensure that maintenance and emergency personnel who may come in contact with dyes and solvents are aware of hazards in order for them to take appropriate precautions by posting Caution Placards at entrances to work areas.
- Personal Protective Equipment (PPE). Skin and eye contact shall be prevented. The following PPE should be worn when handling these materials. Additional information may be found in Work Process I, Personal Protective Equipment.
- At a minimum, safety glasses with side shields, laboratory coats (coveralls are acceptable in shop settings), and closed-toe shoes will be worn when handling these materials. This is to be considered as minimum protection and must be upgraded if necessary.
- Additional PPE such as chemical goggles, face shields, chemical aprons, disposable coveralls, chemically resistant gloves, and respiratory protection must be worn if there is a greater chance of chemical exposure. An EHS Health and Safety Representative may be contacted for assistance in selecting appropriate gloves and respiratory protection. The use of respiratory protection requires an industrial-hygiene hazard evaluation and a medical clearance, followed by a fit test and training by the Research Support Team.
- Consult Work Process I.6, Eye and Face Protection, for guidance on the selection, uses, and limitations of safety glasses, chemical goggles, and face shields.
- Since many chemicals are skin-absorbers (i.e., agents that readily pass through the skin), it is important to select gloves that are chemically resistant to the material. Consult Work Process I, Personal Protective Equipment. This contains a list of skin-absorbing agents and provides detailed guidance for selecting chemically resistant gloves.
- Gloves must be selected on the basis of their chemical resistance to the material(s) being handled, their suitability for the procedures being conducted, and their resistance to wear as well as temperature extremes. Improper selection may result in glove degradation, permeation of the chemical through the glove, and ultimately personal exposure to the chemical. This is a potentially serious situation. Consult Work Process I.5, Gloves, for guidance on the selection, uses, limitations, and disposal of chemically resistant gloves. An EHS Health and Safety Representative may also be contacted for assistance in selecting appropriate gloves.
Table O-1. Glove Selection Table – Glove Material Type1
| Solvents | Neoprene | Butyl | PVC | Nitrile | Natural Rubber | Viton |
| Benzyl alcohol | OK | OK | — | — | — | OK |
| Dimethyl sulfoxide (DMSO) | — | OK | — | — | — | — |
| Ethanol (ethyl alcohol) | — | OK | — | — | — | — |
| Ethylene glycol | OK | — | OK | OK | OK | — |
| Ethylene glycol phenyl ether (2-phenoxyethanol) | — | OK | — | OK | — | — |
| Glycerol (glycerin) | OK | OK | OK | OK | OK | OK |
| Methanol (methyl alcohol) | OK | OK | OK | OK | OK | — |
| Propylene carbonate | — | OK | — | OK | — | — |
1 Check vendor chemical resistance data BEFORE selecting and buying gloves. DO NOT use unless vendor data demonstrates gloves are acceptable even if they are marked “OK” in this table. Gloves are available from VWR Scientific and other vendors via Berkeley Lab’s Procurement & Property web page. If you have difficulty obtaining gloves or any other type of PPE, contact an EHS Health and Safety Representative.
- Storage
- Consult Work Process K, Chemical Storage, for hazardous-material storage requirements, recommendations, and information on chemical incompatibility. It is recommended that laser dyes be stored separately from solvents. Requirements for storing laser dyes and solvents are provided below.
- Follow the storage guidelines in Work Process N, Specific Controls and Procedures — Flammables and Combustible Liquids if the material is either flammable or combustible.
- Use secondary containment for dye pumps and lines.
- Emergency Procedures
- Consult Work Process V, Emergency Procedures and Equipment, for emergency actions regarding chemical spills and personal exposure to chemicals.
- In addition to these requirements, the following applies to laser dye and solvent spills:
- Never use combustible or reactive materials (such as paper towels) to clean up or absorb spills of laser dye and solvents. Keep an adequate number of appropriate spill kits to meet anticipated needs. These are commercially available through VWR Scientific. Typically, products containing diatomaceous earth are used for absorbing organic solvents.
- An emergency eyewash and safety shower should be located in all areas where laser dye and solvents are used. In the event of skin or eye contact, flush the affected area for at least 15 minutes and report to Health Services for evaluation and treatment.
Work Process P. Specific Controls and Procedures — Peroxide-Forming Compounds
- Peroxide Formation.
Peroxide formation in common laboratory chemicals is the result of an autoxidation reaction caused by exposure to air (oxygen). The reaction can be accelerated by exposure to light (UV radiation), heat, or by the introduction of a contaminant. Some organic chemicals have inhibitors added such as BHT (2,6-di-tert-butyl-4-methyl phenol), hydroquinone, or diphenylamine to prevent the accumulation of peroxides by reacting with the peroxide to form a stable product. However, once the inhibitor is consumed, peroxide formation will progress. In addition, some volatile compounds (higher vapor pressure, lower boiling point) present a hazard, since evaporation allows the peroxide to concentrate.
Most organic peroxide crystals are sensitive to heat, shock, or friction, and their accumulation in laboratory reagents has resulted in numerous explosions at other facilities. Additionally, some inorganic compounds are known to form peroxide crystals or mixtures of peroxides/superoxides and decomposition products which may become explosive over time. It is important to identify and manage chemicals that form potentially explosive peroxides before they develop unsafe conditions.
- Peroxide-Forming Compounds
NOTE: Containers of peroxide forming compounds showing visible signs of peroxide crystal formation, discoloration, liquid stratification or leakage; those of unknown age or history (e.g., lack of labeling and testing); those that have exceeded their shelf lives; or containers that have no evidence of testing should not be opened or disturbed. Contact your safety coordinator or the EHS Health and Safety Representative supporting your division for guidance. Upon examining the container, it may be necessary to dispose of it as hazardous waste. The EHS Health and Safety Representative and the EHS Waste Generator Assistant will collaborate with the owner of the material to evaluate it for safe and compliant disposal.
Some common organic moieties can undergo autoxidation to form organic peroxides. Chemical owners should take special care with their management. The following is a list of chemical classes known to form organic peroxides. This information is a resource that can help staff identify organic
peroxide forming chemicals in their areas.
Peroxidizable Organic Moieties
Ordered from Most Likely (1) to Least Likely (14) to Form Peroxides*
| 1. Ethers and acetals with alpha-hydrogen | 6. Vinylalkynes with alpha-hydrogen | 11. Secondary alcohols |
| 2. Alkenes with allylic hydrogen | 7. Alkylalkynes with alpha-hydrogen | 12. Ketones with alpha hydrogen |
| 3. Chloroalkenes, fluoroalkenes | 8. Alkylarenes with tertiary alpha hydrogen | 13. Aldehydes |
| 4. Vinylhalides, esters, ethers | 9. Alkanes and cycloalkenes with tertiary hydrogen | 14. Urea, amides, and lactams with alpha hydrogen atom on a carbon attached to nitrogen |
| 5. Dienes | 10. Acrylates, methacrylates |
*Kelly, R.J., Review of Safety Guidelines for Peroxidizable Organic Chemicals, Chemical Health and Safety, September/October, 1996.
Some common compounds that are known to form peroxides are listed in Table P-1: Peroxide-Former Chemical Groups – Storage Times and Testing Frequencies. This is not an exhaustive list. Activity leads must consult the peroxidizable organic moieties chart (above), SDSs, and other sources of information for chemicals used in their work to determine their peroxide-forming potential. Table P-1 also lists allowable storage times and testing frequencies. Testing is discussed in Section 9.
- Group A of Table P-1 lists chemicals that spontaneously form peroxides on exposure to air without further concentration or evaporation. These materials shall be tested or disposed of within three months of opening unless otherwise specified (testing is discussed in Section 9).
- Group B of Table P-1 lists chemicals that form dangerous peroxides only upon concentration by evaporation or distillation. The materials in this list shall be tested or disposed of within one year of opening the containers.
- Group C of Table P-1 is a representative list of monomers that form peroxides which may act as a catalyst, resulting in explosive polymerization. The materials in this list shall be tested or disposed of within one year of opening the containers.
- For all groups in Table P-1, storage time may be increased to 18 months if the container is unopened and has never been exposed to air/oxygen, unless otherwise specified. After 18 months, the container shall be opened and tested to ensure peroxide levels are within safe limits for continued storage, or must pass the applicable visual inspection criteria for solids (e.g. potassium metal), or must be disposed of.
Table P-1. Peroxide-Former Chemical Groups
– Storage Times and Testing Frequencies
| Group A: Chemicals That Form Explosive Levels of Peroxides Without Concentration(Storage Time After Opening: 3 Months; Testing Frequency: Every 3 Months) | ||||
| Chemical | CAS | Synonyms | State | Note/Reference |
| Butadiene | 106-99-0 | 1,3-Butadiene; Vinylethylene; Biethylene; Divinyl; Methylallene | g | 1, 3, 4, 6 |
| Chloroprene | 126-99-8 | 2-Chloro-1,3- butadiene; β-Chloroprene | l | 1, 3, 4, 5, 6 |
| Divinyl acetylene | 821-08-9 | 1,5-Hexadien- 3-yne | l | 5, 6 |
| Isopropyl ether | 108-20-3 | Diisopropyl ether; 2-Isopropoxypropane | l | 5, 6 |
| Potassium | 7440-09-7 | Potassium metal | s | 8 |
| Potassium amide | 17242-52-3 | Potamide | s | 9 |
| Sodium amide | 7782-92-5 | Sodamide | s | 9 |
| Tetrafluoroethylene | 116-14-3 | Perfluoroethylene; TFE | g | 4, 6 |
| Vinylidene chloride | 75-35-4 | 1,1- Dichloroethylene; 1,1-Dichloroethene;Vinylidene dichloride; VDC | l | 5,6 |
| Group B: Chemicals That Form Explosive Levels of Peroxides on Concentration(Storage Time After Opening: 12 Months; Testing Frequency: Every 12 Months) | ||||
| Chemical | CAS | Synonyms | State | Note/Reference |
| Acetal | 105-57-7 | Acetaldehyde diethyl acetal; Diethylacetyl;1,1-Diethoxyethane | l | 4, 5, 6 |
| Acetaldehyde | 75-07-0 | Ethyl aldehyde; Ethanal | l | 4, 6 |
| Benzyl alcohol | 100-51-6 | Hydroxytoluene; α-Hydroxytoluene; Phenylmethanol | l | 4, 6 |
| 2-Butanol | 78-92-2 | sec-Butyl alcohol; Butan-2-ol | l | 4, 6 |
| Chlorodifluoroethylene | 359-10-4 | 2-Chloro-1,1-difluoroethylene | g | 6 |
| Cyclohexanol | 108-93-0 | Cyclohexan-1-ol; Hexahydrophenol | l | 4 |
| Cyclohexene | 110-83-8 | Tetrahydrobenzene | l | 5, 6 |
| 2-Cyclohexen-1-ol | 822-67-3 | 2-Cyclohexenol | l | 4, 6 |
| Cyclooctene | 931-88-4 | cis-Cyclooctene | l | 5 |
| Cyclopentene | 142-29-0 | l | 5, 6 | |
| Decahydronaphthalene | 91-17-8 | Decalin; Perhydronaphthalene; Bicyclo[4.4.0]decane | l | 4, 6 |
| Diacetylene | 460-12-8 | 1,3-Butadiyne; Biacetylene | g | 4, 5, 6 |
| Dicyclopentadiene | 77-73-6 | 4,7-Methanoindene; 1,3-Cyclopentadiene, dimer; DCPD | l | 4, 5, 6 |
| Diethylene glycol dimethyl ether | 111-96-6 | Diglyme; bis-(2-Methoxyethyl)ether | l | 4, 5, 6 |
| Dioxane | 123-91-1 | 1,4-Dioxane; p-dioxane; Diethylene dioxide;1,4-Dioacyclohexane; Dioxyethylene ether; Glycolethylene ether | l | 4, 5 |
| Ethylene glycol dimethyl ether | 110-71-4 | Glyme; 1,2-Dimethoxyethane; Monoglyme;Dimethyl cellosolve | l | 4, 5 |
| Ethyl ether | 60-29-7 | Diethyl etherl Diethyl oxide; Ethoxyethane;1,1-Oxybisethane | l | 4, 5, 6 |
| Furan | 110-00-9 | Furfuran; Oxole; Oxacyclopentadiene;Divinylene oxide | l | 5, 6 |
| 4-Heptanol | 589-55-9 | Heptan-4-ol | l | 4, 6 |
| 2-Hexanol | 626-93-7 | Hexan-2-ol | l | 4, 6 |
| Isopropyl benzene | 98-82-8 | Cumene; 1-Methylethyl benzene;2-Phenyl propane | l | 5, 6 |
| Methyl acetylene | 74-99-7 | Propyne | g | 4, 5, 6 |
| 3-Methyl-1-butanol | 123-51-3 | Isoamyl alcohol; Isopentyl alcohol;3-Methylbutanol | l | 4, 6 |
| Methyl cyclopentane | 96-37-7 | Methylpentamethylene | l | 5, 6 |
| Methyl isobutyl ketone | 108-10-1 | Methyl-i-butyl ketone; Isopropylacetone; 2-Methyl-4-pentanone; MIBK | l | 4, 6 |
| 4-Methyl-2-pentanol | 108-11-2 | Methyl isobutyl carbinol; Methyl amyl alcohol; 2-Methyl-4-Pentanol | l | 4, 6 |
| 2-Pentanol | 6032-29-7 | sec-Amyl alcohol; sec-Pentyl alcohol;3-Methylbutanol | l | 4, 6 |
| 4-Penten-1-ol | 821-09-0 | 2-Allylethyl alcohol | l | 4, 6 |
| 1-Phenylethanol | 98-85-1 | α-Methyl-benzyl alcohol; Styrene alcohol | l | 4, 6 |
| 2-Phenylethanol | 60-12-8 | Phenethyl alcohol; Phenylethyl alcohol;β-Hydroxyethylbenzene | l | 4, 6 |
| 2-Propanol | 67-63-0 | Isopropanol; Isopropyl alcohol; IPA | l | 4, 6 |
| Tetrahydrofuran | 109-99-9 | 1,4-Epoxybutane; Butylene oxide; Diethylene oxide; Cyclotetramethylene oxide; THF; Furanidine | l | 5, 6 |
| Tetrahydronaphthalene | 119-64-2 | Tetralin | l | 5 |
| Group C: Chemicals That May Autopolymerize as a Result of Peroxide Accumulation(Safe Storage Time After Opening: Inhibited Chemicals, 12 Months; Uninhibited Chemicals, 24 Hours) | ||||
| Chemical | CAS | Synonyms | State | Note/Reference |
| Acrylic acid | 79-10-7 | 2-Propenoic acid; Vinylformic acid | l | 2, 5 |
| Acrylonitrile | 107-13-1 | Acrylon; Cyanoethylene; Vinyl cyanide; Propenenitrile | l | 2, 4 |
| Butadiene | 106-99-0 | 1,3-Butadiene; Vinylethylene; Biethylene; Divinyl; Methylallene | g | 1, 3, 5, 6 |
| Buten-3-yne | 689-97-4 | Vinyl acetylene; Butenyne | g | 4 |
| Chloroprene | 126-99-8 | 2-Chloro-1,3-butadiene; β-Chloroprene | l | 1, 3, 5 |
| Chlorotrifluoroethylene | 79-38-9 | 1-Chloro-1,2,2-trifluoroethylene; CTFE | g | 5 |
| Methyl methracrylate | 80-62-6 | MMA monomer; 2-Methylacrylic acid methyl ester | l | 2, 5 |
| Styrene | 100-42-5 | Vinyl benzene; Cinnamene; Ethenylbenzene | l | 5 |
| Tetrafluoroethylene | 116-14-3 | Perfluoroethylene; TFE | g | 5 |
| Vinyl acetate | 108-05-4 | Ethenyl ethanoate; Ethenyl acetate; Acetic acid vinyl ester | l | 5 |
| Vinyl chloride | 75-01-4 | Mono-chloroethylene; Chloroethene; Ethylene monochloride; VCM | g | 5 |
| Vinylidene chloride | 75-35-4 | 1,1-Dichloroethylene; 1,1-Dichloroethene;Vinylidene dichloride; VDC | l | 5 |
Table P-1 Notes/References:
1. When stored as a liquid monomer.
2. Although these form peroxides, no explosions involving these monomers have been reported.
3. Also stored as a gas in gas cylinders.
4. Kelly, R.J., Review of Safety Guidelines for Peroxidizable Organic Chemicals, Chemical Health and Safety, September/October, 1996.
5. National Research Council, Prudent Practices in the Laboratory, Handling and Disposal of Chemicals; National Academy Press; Washington, D.C., 2011.
6. Clark, D.E., Peroxides and Peroxide-Forming Compounds, Chemical Health and Safety, September/October, 2001.
7. Potassium metal stored under oil has a visual inspection frequency of 1 year after opening. Consult Section 9 for details. If stored under oil in ambient conditions, the maximum total storage time is 5 years from the date of receipt, regardless of the degree of peroxidation. Potassium metal stored under inert atmosphere or vacuum does not have a maximum total storage time, but shall be inspected annually to verify storage conditions.
8. These chemicals are restricted items and require EHS approval prior to purchasing. Maximum total storage time is 3 months after date of receipt.
- Control Measures and Worker Authorization
- Activity leads shall identify peroxide-forming compounds used in their work areas. Sources including this work process, SDSs, and the LBNL Chemical Management System may be consulted. The Chemical Management System is based on the list in Table P-1 and is not an exhaustive method of identifying peroxide-forming compounds.
- The use of peroxide-forming chemicals shall be added to a Work Planning and Control Activity.
- Workers who use peroxide forming compounds shall be authorized by Activity Leads through the WPC system.
- Training and Information
- Employees who manage, handle, or use peroxide-forming chemicals, must complete Chemical Hygiene and Safety Training (EHS0348; or 345 for Facilities personnel) and Managing Time-Sensitive Chemicals (EHS0394).
- Workers who handle peroxide forming compounds shall be given On The Job Training (OJT) in the specific hazards and controls of the materials being handled. This includes the use of peroxide test strips for those responsible for peroxide forming chemical management (see Section 9). OJT is a responsibility of the Activity Lead and Project Lead and is part of the work authorization process for applicable activities. EHS Health and Safety Representatives are available to provide assistance.
- Consult Work Process Y, Container Labeling, for labeling requirements for primary and secondary containers. Additional labeling requirements to track peroxide-forming chemicals test results are discussed in Section 9.
- Entrances to work areas shall be posted with a Caution Placard depicting area hazards and emergency contact information.
- Chemical Inventory Management
- Identify and use safer chemical alternatives (e.g., chemicals that don’t form peroxide crystals) if possible. Otherwise, procure chemicals that have a peroxide inhibitor added (e.g., BHT).
- Limit what you buy or borrow what you need from a colleague in your group or contact the Chemical Management System Coordinator (cms@lbl.gov) to assist you in finding a source of the chemical at Berkeley Lab.
- Conduct periodic cleanouts to prevent accumulating unneeded chemicals.
- Procure and use the minimum amount of material required for the operation. Don’t stockpile chemicals.
- Enter these materials into the Chemical Management System (CMS). Tracking splits of peroxide-forming chemicals is required, if stored, to ensure that time-sensitive hazards are mitigated. Process Guidance for Splits
- Engineering Controls
- A fume hood or other appropriate exhaust ventilation must be used when handling peroxide-forming chemicals in a manner that may produce airborne vapors. This includes procedures such as transfer operations, preparation of mixtures, blending, sonification, spraying, heating, evaporation, and distilling.
- Keep the fume hood sash in its lowest practical position when handling these compounds. If the hood is equipped with one, use the horizontal sliding sash.
- Place safety shields in front of reaction vessels, distillation columns, and other apparatuses when fire, explosion, or detonation may occur.
- Leave at least 10% bottoms when distilling peroxide-forming chemicals.
- All chemicals listed in Table P-1 that have not been stored under an inert atmosphere shall be tested for peroxides (see Section 9) prior to the start of any process that involves distilling or concentrating the compound.
- Personal Protective Equipment (PPE). Skin and eye contact must be prevented. The following PPE should be worn when handling these materials (Additional information may be found in Work Process I, Personal Protective Equipment):
- At a minimum, safety glasses with side shields, long pants, laboratory coats (coveralls are acceptable in shop settings), and closed-toe shoes will be worn when handling these materials. This is to be considered as minimum protection and must be upgraded if necessary.
- Additional PPE such as chemical goggles, face shields, chemical aprons, disposable coveralls, chemically resistant gloves, and respiratory protection must be worn if there is a greater chance of chemical exposure. An EHS Health and Safety Representative may be contacted for assistance in selecting appropriate gloves and respiratory protection. The use of respiratory protection requires a hazard evaluation and a medical clearance followed by a fit test and training by the EH&S Division Research Support Team.
- Consult Work Process I.6, Eye and Face Protection, for guidance on the selection, uses, and limitations of safety glasses, chemical goggles, and face shields.
- Since many chemicals are skin-absorbers (i.e., agents that readily pass through the skin), it is important to select gloves that are chemically resistant to the material. Consult Work Process I.5, Gloves. This contains a list of skin-absorbing agents and provides guidance for selecting chemically resistant gloves.
- Gloves must be selected on the basis of their chemical resistance to the material(s) being handled, their suitability for the procedures being conducted, and their resistance to wear as well as temperature extremes. Improper selection may result in glove degradation, permeation of the chemical through the glove, and ultimately personal exposure to the chemical. This is a potentially serious situation. Consult Work Process I.5, Gloves, for guidance on the selection, uses, limitations, and disposal of chemically resistant gloves. An EHS Health and Safety Representative may also be contacted for assistance in selecting appropriate gloves.
- Storage
- Consult Work Process K, Chemical Storage, for hazardous materials storage requirements, recommendations, and information on chemical incompatibility. Additional requirements for peroxide forming compounds are provided below.
- Follow the storage guidelines in Work Process N, Specific Controls and Procedures — Flammables and Combustible Liquids, if the material is a flammable or combustible liquid. Follow the storage guidelines in Work Process Q. Specific Controls and Procedures — Water-Reactive Chemicals, if the material is a water-reactive chemical (e.g. potassium metal).
- Refer to Table P-1 for allowable storage times. Peroxide-forming compounds may be stored for the indicated time periods after opening. Storage for longer periods of time is allowable for organic liquids provided that testing results are within acceptable concentration limits (see Section 9 for peroxide testing and labeling).
- Store peroxide-forming chemicals in sealed, air-impermeable containers. Dark amber glass containers with tight-fitting caps are required. Do not use containers with loose-fitting lids or glass stoppers. These may allow the introduction of air and result in peroxide formation.
- Septum-capped containers such as Sure Seal® bottles and air-free transfer techniques such as with a Schlenk line minimize entrainment of air.
- Storage in inert glove boxes provides an added measure of safety since there is no contact with air.
- Peroxide Testing and Labeling
NOTE: Containers of peroxide forming compounds showing visible signs of peroxide crystal formation, discoloration, liquid stratification or leakage; those of unknown age or history (e.g., lack of labeling and testing); those that have exceeded their shelf lives; or containers that have no evidence of testing should not be opened or disturbed. Contact your safety coordinator or the EHS Health and Safety Representative supporting your division for guidance. Upon examining the container, it may be necessary to dispose of it as hazardous waste. The EHS Health and Safety Representatives and the Waste Management Generator Assistant will collaborate with the owner of the material to evaluate it for safe and compliant disposal.
- The following concentration limits are used at LBNL:
- If the peroxide concentration is less than 100 ppm, the chemical may be used, but DO NOT DISTILL OR CONCENTRATE if the concentration is greater than or equal to 30 ppm.
- If the peroxide concentration is greater than or equal to 20 ppm, the material shall be evaluated prior to disposal. Contact your EHS Waste Generator Assistant for guidance.
- Peroxide-forming organic liquids shall be tested for peroxides at least annually following Section 9c. Peroxide-forming gases or solids are not testable using peroxide test strips, but shall be visually inspected at least annually for the failure criteria noted above. The following exceptions apply to periodic testing as outlined in Section 9c:
- Grignard reagent–peroxide former solutions: Grignard reagents inhibit the formation of peroxides. Generally, periodic testing of these solutions is not needed especially for those stored in inert glove boxes and/or septum-capped containers such as Sure Seal® bottles. Evaluation and testing is required prior to waste disposal for chemicals on the Waste Management Peroxide-Forming List. Contact the EHS Waste Generator Assistant for guidance.
- Unopened, intact containers do not have to be opened for the purpose of periodic testing unless they have reached their storage time limit per Table P-1 or are to be disposed of as waste. Evaluation and testing is required prior to waste disposal for chemicals on the Waste Management Peroxide-Forming List. Contact the EHS Waste Generator Assistant for guidance.
- Containers stored in inert atmosphere glove boxes do not require periodic testing since they have had no contact with oxygen. Evaluation and testing is required prior to waste disposal for chemicals on the Waste Management Peroxide-Forming List. Contact the EHS Waste Generator Assistant for guidance.
- Peroxide-forming compounds in septum-capped containers such as Sure Seal® bottles, do not require periodic testing provided that they have had no contact with air. Note: Repeated puncturing of a septum capped container, or failure to backfill it with an inert gas may introduce air into the container. Evaluation and testing is required prior to waste disposal for chemicals on the Waste Management Peroxide-Forming List. Contact the EHS Waste Generator Assistant for guidance.
- Potassium metal: Potassium metal shall be visually inspected for evidence of peroxide formation on an annual basis once opened. Potassium peroxide formation includes yellow, orange or black discoloration, spots, or film formation. If peroxide formation is suspected or observed, discontinue use and contact your EHS Waste Generator Assistant for guidance. White oxide layers or film formation are typical and do not indicate presence of peroxides.
- Peroxide Testing Method
- Use commercially available peroxide test strips such as Quantofix® Peroxide 100, or EM Quant® Peroxide Test Strip, 0-100 mg/l. These are available via EHS using the request form or via the Laboratory’s Procurement & Property website. (Note: the units: “mg/l” and “ppm” are equivalent in solution concentrations). These test strips contain the enzyme peroxidase, which transfers oxygen from the peroxide to an organic redox indicator, which is then converted to a blue oxidation product. Carefully follow the manufacturer’s instructions for testing and interpreting results.
- The test strip must be treated with a drop of distilled water after the solvent has evaporated, otherwise the test will produce a false negative.
- Peroxide Test Label
- Peroxide-forming chemicals, including splits of peroxide-forming chemicals if stored, shall be labeled to clearly identify to all trained personnel that the chemical may form explosive peroxides, and the container shall be marked with the date the container was received, the date first opened, and the testing interval (see Table P-1). Additionally, a complete record of test results (in ppm, or pass/fail for visual inspection of potassium metal) and the dates of the tests shall be recorded and kept until the container has been properly disposed. The chemical owner or designee shall enter test records into the CMS database. This ensures test history will remain accessible regardless of personnel changes and that CMS testing notifications will be provided.
- It is strongly recommended to also keep this record either on the peroxide-forming chemical label, or in a separate, easily accessible log.If the chemical owner opts for a separate, easily accessible log separate from CMS, then the results shall be indexed to the unique CMS RFID tag of the container(s), and this log must be in a format and location (be it physical or digital) that will remain accessible regardless of personnel changes.
- EHS provides an official label for peroxide-forming organic chemicals, which may be obtained from the EHS Health and Safety Representative supporting your Division or via the request form. Any alternative labels that a chemical owner wishes to use must be approved by the owner’s Division Safety Coordinator and the EHS Research Support Team. This label should be affixed to the container shortly after purchase. Do not wait for the first test date to attach this label.
- The following concentration limits are used at LBNL:
- Disposal
Note: The requirements of Section 9 must be implemented before initiating the disposal process described in this section.
Disposal criteria apply to all hazardous waste mixtures containing ≥ 20% peroxide-forming compounds.
Contact your Safety Coordinator or the EHS Health and Safety Representative supporting your division if you have questions regarding safety. Waste disposal questions should be addressed to the EHS Waste Generator Assistant.
After evaluation and testing, disposal of peroxide forming compounds shall be coordinated through the EHS Waste Management Group. This is to ensure the handling and disposal process meets Treatment, Storage, Disposal Facility (TSDF) waste acceptance criteria (WAC) to dispose of hazardous wastes. WAC for offsite vendor TSDFs include requirements for testing peroxides in mixtures and non-mixtures for chemicals on the LBNL Waste Management Peroxide-Forming Chemical Test.
- Submit a peroxide-forming chemical evaluation form to the Waste Management Group when disposing of chemicals on the Waste Management List of Peroxide-Forming chemicals, and for hazardous waste mixtures containing ≥ 20% total volume of these peroxide-forming chemicals. The peroxide test must be performed within no more than 30 days prior to submission of the hazardous waste requisition.
- Peroxide-forming compounds under 20 ppm can be requisitioned as routine hazardous waste through the electronic hazardous waste management system. Make arrangements for disposal of hazardous waste prior to exceeding this limit unless the material will be completely consumed or purified for continued use. If your peroxide-forming compound contains 20 ppm or greater, an emergency treatment permit and EH&S Division management approval are required for disposal.
- Peroxide-forming compounds containing 20 ppm or greater peroxides cannot be safely disposed of as routine hazardous waste and require disposal through treatment under an Emergency Permit or other immediate external intervention. EHS division management approval is required. Do not attach a hazardous waste label, requisition, or place hazardous waste of peroxide-forming compounds containing 20 ppm or greater into any SAA. Contact your Division’s Generator Assistant for assistance.
- Peroxide-forming compounds are safe for continued use under 100 ppm following the requirements in this work process. If the peroxide-forming compound contains 100 ppm or greater peroxides, immediately discontinue use of the container and contact your Division Safety Coordinator or Health and Safety representative from EHS to initiate a risk assessment. At a minimum, EH&S Division management approval is required for disposal of peroxide-forming compounds with 100 ppm or greater peroxides.
Work Process P.1 Specific Controls and Procedures — Additional Time-Sensitive Chemicals
This work process outlines the requirements for the safe management of time-sensitive chemicals other than peroxide-forming chemicals. All owners of time-sensitive chemicals must follow the policy provided herein, including: tracking all containers of time-sensitive chemicals in the Chemical Management System; labeling all containers of time-sensitive chemicals; assessing all containers of time-sensitive chemicals on at least an annual basis; and working with EHS and Waste Management to safely manage and/or dispose of expired, unsafe or unneeded time-sensitive chemicals.
The work process is organized as follows:
- Section 1 gives a brief overview of what time-sensitive chemicals are and why they must be managed carefully.
- Section 2 identifies the key responsibilities for employees and affiliates at all levels who are involved with the use of time-sensitive chemicals.
- Section 3 lists the required training for those who work with time-sensitive chemicals.
- Section 4 describes how to properly track time-sensitive chemicals using the Chemical Management System.
- Sections 5 and 6 outline the requirements for engineering controls and personal protective equipment, respectively.
- Sections 7 through 10 provide the detailed policy for the labeling, assessment and handling of four categories of time-sensitive chemical: unstable/self-reactive with production of gaseous products; hazardous polymerization; explosive when dry; and time-sensitive gases.
- Section 11 details the process for getting rid of time-sensitive chemicals that are no longer needed, have passed their expiration dates, or are no longer safe to keep. Please note: do not place any time-sensitive chemicals into a satellite accumulation area (SAA) until they have been evaluated by your EHS Health and Safety Representative and the Waste Management Team.
- Section 12 contains some relevant Lessons Learned and incident reports involving time-sensitive chemicals.
- Time-Sensitive Chemicals
Time-sensitive chemicals can develop additional hazards during storage, even if stored and handled properly. Peroxide-forming organic chemicals are only one class of time-sensitive chemical, and this work process pertains to four other classes of time-sensitive chemicals.
It is important to recognize the potential hazards and the innate unpredictability of these chemicals. The rate at which a time-sensitive chemical becomes dangerous to handle depends on many factors. Even when stored properly, if ignored or forgotten a time-sensitive chemical can become unstable, unsafe or potentially explosive. When stored improperly or mishandled, time-sensitive chemicals can pose an immediate danger in the research environment.
The Environment, Health & Safety Division is available to provide assistance in identifying time-sensitive chemicals and determining the best storage conditions and other controls for storing and handling these materials. Contact your Health and Safety Representative or the Time-Sensitive Chemicals Subject Matter Expert (SME) for help.
- Requirements and Responsibilities
The chemical owner is ultimately responsible for the management of his or her time-sensitive chemical inventory. Responsibilities are assigned as follows:
Table 2.1 Responsibilities for the Management of Time-Sensitive Chemicals
| Time-Sensitive Chemicals Subject Matter Expert | Maintain this policy with up-to-date and accurate information.Assist with the continual identification of time-sensitive chemicals.Remind chemical owners of the time-sensitive chemicals in their inventories and of the need to label, track and regularly assess containers of time-sensitive chemicals.Assist with the safe management of expired or unneeded time-sensitive chemicals, as necessary. |
| Operations Staff | Evaluate stability of improperly managed time-sensitive chemicals; summary of roles and responsibilities for relevant Operations Staff can be found here. Note: these roles and responsibilities apply to Work Process P (management of peroxide-forming compounds) and Work Process P.1 (additional time-sensitive chemicals). |
| Chemical Owner | Identify time-sensitive materials owned by you.Know the correct storage conditions and safe handling procedures for all time-sensitive chemicals that you are responsible for.Ensure that containers of time-sensitive materials are stored appropriately according to manufacturer’s instructions, SDS information, and/or other relevant sources.When time-sensitive materials are identified that are not categorized as such in the Chemical Management System, notify the CMS manager at cms@lbl.gov of the discrepancy.Ensure that all containers of time-sensitive materials are |
| Designated Responsible Person(s) | Perform the duties of the chemical owner, as assigned, with the delegated authority of the chemical owner. |
| Activity Lead | Identify time-sensitive materials used in the Activity.Maintain current description of work, hazards and controls in Activity Manager, and update the description of work whenever the scope of work changes.Provide, or designate a knowledgeable and authorized person to provide, on-the-job training to workers for all of the time-sensitive chemicals that the given worker will handle.Ensure that all workers know how to find the assessment records for time-sensitive chemicals.Determine the competency of workers to perform the work described by the Activity, and authorize workers in Activity Manager as appropriate.Notify the Chemical Owner or Designated Responsible Person of any label discrepancies, abnormal conditions, or lapses in the management of time-sensitive chemicals that are found.When time-sensitive materials are identified that are not categorized as such in the Chemical Management System, notify the CMS manager at cms@lbl.gov of the discrepancy. |
| Worker | Identify time-sensitive materials used in the course of work.Receive appropriate on-the-job training for time-sensitive chemicals.Maintain current training in Chemical Hygiene and Safety (EHS 0348 and refresher course EHS 0353) |
- Training and Additional Information
- Employees who manage, handle, or use time-sensitive chemicals must complete EHS 0348, Chemical Hygiene and Safety Training (or EHS 0345 for Facilities personnel), and complete the Chemical Hygiene and Safety Training Refresher course, EHS 0353, every three years thereafter, and Managing Time-Sensitive Chemicals (EHS0394).
- Employees who handle time-sensitive chemicals must also read and be familiar with this Work Process and receive on-the-job training (OJT) in the hazards, controls and storage requirements for the specific materials being handled.
- Information on the identification of time-sensitive chemicals may be found in Sections 7-10 for each category of time-sensitive material.
- For information regarding the management of time-sensitive chemicals in general, see Management of Time-Sensitive Chemicals, parts (I) through (III), in the Journal of Chemical Health and Safety authored by Jim Bailey et al (parts I and II) and David Quigley et al (part III)1.
- Employees who handle time-sensitive chemicals are also highly encouraged to consult Bretherick’s Handbook of Reactive Chemical Hazards Sixth or Seventh Edition, Volume 2, and the associated references in Volume 1 of the same and in the literature. A copy of both volumes is available for your reference with the Time-Sensitive Chemical Subject Matter Expert.
- More specific references for each category of time-sensitive chemical are provided where relevant throughout the work process.
1 Bailey et. al. Management of time sensitive chemicals (I): Misconceptions leading to incidents. Chem. Health Safe. 2004, 11(5), 14-17.
Bailey et. al. Management of time sensitive chemicals (II): Their identification, chemistry and management. Chem. Health Safe. 2004, 11(6), 17-24.
Quigley et. al. Management of time sensitive chemicals (III): Stabilization and treatment. J. Chem. Health Saf. 2006, 13(1), 24-29.
- Chemical Inventory Management
- All time-sensitive chemicals must be entered into the Chemical Management System (CMS) for chemical inventory tracking. The chemical owner or designated responsible person shall ensure that all containers of time-sensitive chemicals, whether in the manufacturer’s container or having been transferred in whole or in part to a new container, are entered into CMS with all relevant information including the date of acquisition of the original container.
- Exceptions
- Containers that are in use and will be emptied or disposed at the end of the experiment or procedure.
- Also see Section 8 for quantity exceptions for chemicals that undergo hazardous polymerization.
- Best Practices
- Before purchasing a time-sensitive chemical, it may be beneficial to use a small amount from another research group to test the procedure and ensure that the chemical will meet the research needs.
- Time-sensitive chemicals should be purchased in the smallest practical quantities to avoid accumulation of leftover or unneeded material.
- Periodic chemical inventory clean-outs can help reduce accumulation of time-sensitive chemicals. A recommended time period is every 2-3 years.
Note: LBNL policy for chemical inventory tracking does not generally require materials transferred out of the original manufacturer’s container to be RFID tagged
and entered in the Chemical Management System. However, due to the inherent dangers of time-sensitive chemicals, every container (including bottles, jars, vials, etc.) must be
RFID tagged
and tracked
in CMS
if it is to be stored.
- Engineering Controls. Engineering controls for the use of time-sensitive chemicals shall be selected based on the hazards of the particular substance(s).
- See Work Process H for general information on engineering controls. EHS is available to assist with selection of proper engineering controls.
- The following work processes may be consulted for more information on controls for handling chemicals with specific hazards:
- Work Process L, Acids and Bases
- Work Process M, Particularly Hazardous Substances: Carcinogens, Reproductive Toxins, and Acute Toxins
- Work Process N, Flammables and Combustible Liquids
- Work Process Q, Water-Reactive Chemicals
- Work Process R, Pyrophoric Materials
- Work Process T, Chemicals with Explosive Properties
- Personal Protective Equipment
- Minimum personal protective equipment when working with time-sensitive chemicals consists of:
- Long pants
- Closed toe shoes
- Safety glasses with side shields
- A laboratory coat
- Chemically resistant gloves
- Consult the Safety Data Sheet of the particular chemical and/or manufacturer glove compatibility literature, for the appropriate glove type(s).
- See Work Process I, Personal Protective Equipment for more information on selection of appropriate PPE, including the use of cover goggles, face shields, aprons and chemically resistant sleeves. EHS is also available to assist with proper PPE selection.
- Minimum personal protective equipment when working with time-sensitive chemicals consists of:
- Unstable/Self-Reactive with Production of Gaseous Products
- Description of Hazard. Some chemicals self-react or decompose to generate heat and gaseous products 2. In some cases, the products of the self-reaction also accelerate the reaction 3. Even if these chemicals do not undergo thermal runaway due to the generation of heat, the constant buildup of gas inside the container can lead to eventual over-pressurization and violent container rupture. The introduction of moisture into chemicals of this type will often initiate or accelerate the decomposition or self-reaction.
- Identification. Specific chemicals and chemical categories that are known to self-react to produce gaseous products are listed, with examples, in Tables 7-1 and 7-2. Many of these undergo autocatalytic decomposition or condensation reactions.
- Storage
- Many chemicals in this category are stored cold to slow the self-reaction or decomposition. However, not every manufacturer’s SDS contains complete storage information. It is recommended that owners of this category of time-sensitive chemical check multiple SDS documents and any other available sources to ensure complete information on the storage requirements.
- Most of the chemicals in this category are also sensitive to contamination, which will increase the rate at which they react or decompose to produce gas.
- Many are water-reactive (especially the chloroformates, furoyl halides and thiophene carbonyl halides) and even a small amount of moisture may cause a rapid buildup of pressure in the container.
- Labeling, Assessment and Venting
- All containers of this class of time-sensitive chemical shall bear a label identifying them as a time-sensitive chemical. Additionally, the Designated Responsible Person must keep CMS testing records up to date that contain:
- Date the container was opened
- Inspection frequency
- Date and result of all inspections (
Note: It is strongly recommended to also keep testing records either on the label or in an easily accessible log.
)
- Once opened, this classification of time-sensitive chemicals shall be assessed on a schedule commensurate with its hazard as communicated by the manufacturer, in the SDS, in the relevant literature, and/or in the experience of the research group.
- The assessment period in any case shall not be greater than one year. In many cases, the assessment period for chemicals of this type should be significantly less than one year. For help determining an appropriate assessment period, contact the Time-Sensitive Chemical subject matter expert.
- During each assessment, the container shall be carefully vented. Note that the presence of a vented cap on the container does not exempt the container from this requirement.
- A lab coat and appropriate chemical resistant gloves must be worn while venting containers.
- This procedure shall be performed in a fume hood or glove box if appropriate.
- The bottle is opened slowly and carefully to allow excess pressure to escape, then re-closed.
- Venting of air- or water-sensitive chemicals supplied with a septum cap may be accomplished using needles. A low pressure inert gas supply may be used to prevent backflow of air into the container.
- The safety of a container in this classification shall be assessed visually by checking for the following failure criteria:
- Prolonged abnormal storage conditions such as storage at room temperature when the SDS or manufacturer’s instructions indicate cold storage;
- Any evidence of pressure build-up in the container, such as a bulging cap;
- The container has passed the expiration date provided by the manufacturer.
- Any time-sensitive material container that fails any one of these criteria shall be considered unsafe or expired, and the procedure in Section 11 shall be followed for the safe management and disposal of the container. (Note: If the container has yet to be opened, do not open it to vent or assess it. The periodic assessment cycle commences when the container is first opened.)
- All containers of this class of time-sensitive chemical shall bear a label identifying them as a time-sensitive chemical. Additionally, the Designated Responsible Person must keep CMS testing records up to date that contain:
- Handling Considerations
- Vent previously opened containers frequently, especially if they are rarely used.
- Heating this class of chemicals may increase the decomposition rate drastically or lead to a thermal runaway. Carefully consider all reaction conditions before heating this class of chemical, including conditions for safely venting any gaseous products that may result.
- Store containers with vented caps in a fully upright position to prevent leaks. The presence of a vented cap does not exempt the container from regular assessment and venting of previously opened containers.
- Ensure that all tools used, such as spatulas or pipettes, are clean and dry. This class of chemical is particularly sensitive to contamination, which includes moisture in many cases.
- Never return unused portions of the chemical to the original container.
- Do not mix the chemical with any incompatible materials when disposing of unused chemical portions or mixtures containing unused chemicals in this category. This may lead to pressure buildup in the waste container. It may be safest to use a dedicated container with no other materials.
- Metal containers and caps can be a source of contamination, and some of the materials in this class are initiated or catalyzed by metals and/or metal oxides such as iron or rust.
- Hazardous Polymerization
- Description of Hazard. Many monomers are supplied with an inhibitor added to prevent polymerization until the experimenter intentionally initiates it. This leads to greatly prolonged shelf lives of monomers that may otherwise polymerize, sometimes violently, in a short period of time. However, the inhibitors used are often sacrificial by nature, and thus during prolonged storage and exposure to adverse conditions the inhibitor concentration may decrease over time. Eventually the inhibitor concentration may decrease to the point that it is no longer effective and polymerization will begin. Not only does this lead to a useless reactant, but the reaction is usually exothermic and accelerates with increasing temperature, which can produce considerable heat and rupture containers with significant force4. This process is known as thermal runaway. The potential for violent polymerization largely depends on the nature of the chemical, but for any one substance the risk is increased with increasing container size. Industrial accidents involving runaway polymerizations of stored material are frequent and sometimes deadly to those involved5. Accidents of this nature rarely happen on the laboratory scale because small containers are capable of releasing heat to the environment faster than their internal volume can produce heat by reaction. Often, small bottles will simply polymerize over time with no detrimental effects. However, on a larger scale the risks are well documented, including fires and explosions resulting in severe injury and loss of life. Therefore, any production-scale containers of materials that can undergo hazardous polymerization upon consumption of the inhibitor must be carefully tracked and managed to prevent accidents.
- Identification
- Small volumes of the chemicals in this category pose very little risk of accident. Therefore, only production-scale quantities of this category are considered time-sensitive for the purposes of this policy. Researchers should consider the special considerations (reactivity, exothermic properties, quantity) of the chemicals that they are using and determine if and how the chemicals should be managed. EHS can assist with this determination. Research-scale quantities of monomers with a hazardous polymerization hazard may be handled, stored and tracked in the same manner as chemicals that are not time-sensitive.
- There is some overlap between chemicals that undergo hazardous polymerization and peroxide forming organic chemicals. Both the formation of organic peroxides and the initiation of hazardous polymerization start with the homolytic cleavage of a weak C-H bond to form a radical. In the presence of oxygen, this radical may combine with dissolved oxygen to produce an organic peroxide. In the absence of oxygen, the radical may initiate polymerization. For more information, see Work Process P: Specific Controls and Procedures – Organic Peroxide-Forming Compounds. A list of chemicals that are known or suspected to have undergone violent polymerization can be found here. It is not exhaustive.
- Some of the most common chemical categories that undergo hazardous polymerization are included in Table 8.1.
- The most commonly used chemicals at LBNL that undergo violent polymerization in production-scale quantities are shown in Table 8.2.
4 Polymerization Incidents. Bretherick’s Handbook of Reactive Chemical Hazards, 6th Edition, Volume 2, pages 324-327.
Violent Polymerization. Bretherick’s Handbook of Reactive Chemical Hazards, 6th Edition, Volume 2, pages 397-398
5 Calorimetric evaluation of polymerization thermokinetics of styrene, -methylstyrene and trans-methylstyrene. S.Y. Lin, K.Y. Chen, C.M. Shu, Journal of Hazardous Materials 161 (2009) 330-335.
- Storage. The manufacturer’s instructions (if provided) and the SDS must always be followed where they conflict with or are more specific than the general guidance offered here.
- Storage Temperature: This class of time-sensitive chemicals is generally stored cold but not frozen.
- Storage Containers: Many of this class of material are light-sensitive, requiring storage in either opaque containers or amber glass bottles.
- Other Considerations – Atmosphere: The gas occupying the headspace of a container can modify the behavior of chemicals in this category. In the absence of dissolved oxygen, the conversion of carbon radicals to peroxides is not possible, and the formation of radicals is more likely to result in the initiation of hazardous polymerization 6. However, with the presence of dissolved oxygen, these chemicals may accumulate organic peroxides and may need to be managed according to Work Process P: Specific Controls and Procedures – Organic Peroxide-Forming Compounds.
6 Acrylic Monomers. Bretherick’s Handbook of Reactive Chemical Hazards, 6th Edition, Volume 2, page 5.
- Assessment and Labeling
- Production-scale quantities of this classification of time-sensitive chemical shall be assessed on a schedule commensurate with their hazard as communicated by the manufacturer, in the SDS, and/or in the relevant literature.
- The assessment period in any case shall not be greater than one year.
- The safety of a container in this classification shall be assessed visually by checking for the following failure criteria:
- Prolonged abnormal storage conditions such as storage at room temperature when the SDS or manufacturer’s instructions indicate cold storage;
- Any evidence of pressure build-up in the container, such as a bulging cap;
- Any evidence of polymerization, such as solid residue in the container;
- The container has passed its expiration date as provided by the manufacturer.
- Any time-sensitive material container that fails any one of these criteria shall be considered unsafe or expired, and the procedure in Section 11 shall be followed for the safe management and disposal of the container.
- All containers of this class of time-sensitive chemical shall bear a label identifying them as a time-sensitive chemical. Additionally, the Designated Responsible Person must keep CMS records up to date that contain:
- Date the container was opened
- Inspection frequency
- Date and result of all inspections
Note: It is strongly recommended to also keep testing records either on the label or in an easily accessible log.
- Handling Considerations
- Heating this class of chemicals may lead to violent polymerization and thermal runaway, which may release toxic vapors, over pressurize and rupture containers, or ignite fires.
- Avoid freezing of liquids, as the frozen domains will exclude the inhibitor and the process of thawing the material may initiate polymerization. As the material thaws, local areas of uninhibited material can initiate a violent reaction, even under mild/ambient heating or thawing conditions. Uneven heating can also cause localized hot spots within the material that can initiate a thermal runaway polymerization.
- Metal containers and caps can be a source of contamination, and some of the materials in this class are initiated or catalyzed by metals and/or metal oxides such as iron or rust.
- Store in a cool, dry, dark place to avoid temperature fluctuations and exposure to light.
- Exercise great care when initiating large-scale polymerization reactions. Long induction periods can lead to an underestimation of the reaction progress and eventual reaction rate. This has caused many industrial accidents. 7
7 Induction Period Incidents. Bretherick’s Handbook of Reactive Chemical Hazards, 6th Edition, Volume 2, pages 183-184.
- Explosive When Dry
- Description of Hazard. This category encompasses substances that are shock-, friction- or heat-sensitive explosives when dry, but which have been stabilized with liquid for safer handling and transportation. If the substance is purchased and/or stored dry, then it falls under the controls in Work Process T, Chemicals with Explosive Properties. While sufficiently wetted or in solution, these materials can be handled without risk of detonation. However, if the liquid or solvent evaporates upon prolonged storage, the sensitive explosive material will be left behind and there is a risk of inadvertent detonation. The liquid used is sometimes quite volatile, such as diethyl ether or ethanol, and can evaporate quickly if containers are not properly resealed. Most reported incidents involving this class of material involve the friction-initiated detonation of dry crystals, either during scraping of the material as with a spatula, or when material has dried in the threads of the cap and the bottle is then opened.
In addition to the risks associated with the material drying out, many of these chemicals also form incredibly sensitive explosives on contact with metals, whether wetted or not. For example, picric acid is only moderately shock sensitive, but metal picrates are incredibly sensitive to shock, friction, heat and electrostatic discharge, making them extremely dangerous to handle even when wetted. It is important to check the SDS thoroughly for incompatibilities and to avoid contaminating the material with anything that might increase its explosive sensitivity. - Identification. The most well-known explosives are polynitro organic compounds and nitrate esters such as nitroglycerin, trinitrotoluene (TNT), nitrocellulose (also known as collodion when wetted) and picric acid (trinitrophenol). However, many other functional groups can confer explosive properties and sensitivity to shock, friction or heat. Please refer to Table 9.1.
- Explosive When Dry Chemicals with Special Handling and/or Additional Controls
- Some specific compounds and categories of compounds are:
- known to be particularly sensitive to shock, friction or heat when dry;
- known to have caused multiple incidents in the laboratory setting; or
- potentially explosive even when wetted.
- These materials must be handled with extreme care and will require more extensive planning and review prior to acquisition. Researchers who wish to order and use these compounds or any chemical in one of these categories should work with their division Health and Safety Representative, Division Safety Coordinator, and Environmental Health and Safety Subject Matter Expert to plan specific controls and safeguards. Refer to Tables 9.2 and 9.3.
- All primary explosives are prohibited at Lawrence Berkeley National Laboratory, whether wetted or not. Primary explosives are incredibly sensitive to heat, shock, friction or electrostatic discharge. These materials are often used to initiate other, less sensitive explosives. Common primary explosives include: acetone peroxide (triacetone triperoxide), diazodinitrophenol (DDNP), hydrazinium perchlorate, hydroxylammonium nitrate, lead azide, lead picrate, ammonium chlorate, silver fulminate, mercury fulminate, nitroglycerin, and pentaerythritol tetranitrate (PETN). Additional primary explosives may be found on the LBNL Restricted Chemicals and Gases List found here. This list is not exhaustive. It is the responsibility of the researchers to determine whether a material is a primary explosive using resources such as SDS documents, literature reports, and manufacturer-provided information. EHS can assist with identification. See Work Process T, Chemicals with Explosive Properties for more information.
- Some specific compounds and categories of compounds are:
- Storage
- Materials that are explosive when dry shall be stored in tightly closed containers in cool, dark locations to minimize evaporation.
- If the liquid or solvent is a flammable or combustible liquid, these materials must be stored in an approved flammable storage cabinet with self-closing, self-latching doors.
- It is recommended that these materials be stored inside a sturdy cabinet away from other materials where they are unlikely to be disturbed when not in use.
- Assessment and Labeling
- The safety of chemicals in this classification shall be assessed by visual inspection for the following failure criteria:
- There is evidence of significant evaporation as indicated by a liquid level marking. For modest evaporation, top up to level marking with appropriate solvent to stabilize material until the next assessment date;
- Solid material has precipitated from solution (in cases where the material is soluble in its stabilizing liquid);
- Crystals or solid material are visible around the bottom of the cap;
- Crystals or solid residue has formed on the outside of the container from dripping or leaking of the contents.
- Any time-sensitive material container that fails any one of these criteria shall be considered unsafe or expired, and the procedure in Section 11 shall be followed for the safe management and disposal of the container.
- Determining an appropriate assessment period for this class of time-sensitive chemical is crucial.
- In some cases, an Explosive When Dry material may be supplied with just enough liquid to stabilize the material. For example, picric acid/picrylsulfonic acid is often supplied in a near-concentrated solution, so any evaporation may lead to crystallization of the explosive material. These materials should have a shorter assessment period, such as one to three months.
- In other cases, an Explosive When Dry material may be supplied with a large excess of solvent, and need only stay wetted or moist to remain stable. For example, nitrocellulose, also known as collodion, is often supplied under a large excess of solvent. The assessment period for these materials can therefore be longer, but may not exceed one year.
- Some factors to consider when determining an appropriate inspection period include:
- Volatility/evaporation rate of the solvent or liquid that stabilizes the material;
- Concentration of the material in the solvent/liquid;
- Whether the material is stabilized by the mere presence of some wetting liquid, or must remain below a critical concentration for stability.
- In any case, the inspection period shall not be greater than 12 months.
- Each container of this class of time-sensitive chemical shall bear a label identifying it as a time-sensitive chemical. Additionally, the Designated Responsible Person must keep CMS testing records up to date that contain:
- Date the container was opened
- Inspection frequency
- Date and result of all inspections
Note 1: If a particularly sensitive explosive, such as diazomethane, is produced in the laboratory in a stabilizing solution or liquid, it shall not be stored for later use. It must be used in its entirety or quenched at the end of the process.Note 2: It is strongly recommended to also keep testing records either on the label or in an easily accessible log.
- The safety of chemicals in this classification shall be assessed by visual inspection for the following failure criteria:
- Handling Considerations
- Check the SDS carefully for incompatibilities that might increase the explosive sensitivity of explosive when dry materials. In particular, some of these materials are incompatible with metals, and care must to be taken to avoid the use of metal containers, lids, linings and tools.
- Never scrape explosive when dry material, even when still wetted. Multiple explosions have occurred during recovery of an explosive when dry material from the filter of a Büchner funnel due to scraping.
- Mark the liquid level on the outside of the bottle of containers of explosive when dry materials to track evaporation so that the liquid can be topped off as necessary. Always use a liquid or mixture with identical composition to the liquid/solvent provided by the manufacturer.
- Wrapping the cap with a gas-impermeable tape, such as electrical tape, may slow the evaporation rate. Note that Parafilm® is manufactured to be permeable to gases and may not significantly slow evaporation.
- Heating an explosive when dry material may cause solvent loss and/or detonation of the material
- Use only tools that are compatible with the material. Some materials are sensitive to contamination with metals and should not be handled with metal tools. Others may be sensitive to static discharge and should not be handled with disposable plastic tools. Teflon coated tools offer the best resistance to both metal contamination and static discharge. However, the teflon coating can become scratched or damaged, revealing the metal beneath. Check tools carefully for compatibility and for defects before use with explosive when dry materials.
- Metal containers, caps and trays can also be a source of contamination. Use plastic caps whenever possible, and avoid storing these containers in metal trays.
- Avoid the use of glass stoppers or ground glass joints. The friction/scraping of ground glass joints can initiate explosions if material dries between the two glass pieces.
- Description of Hazard. This category encompasses substances that are shock-, friction- or heat-sensitive explosives when dry, but which have been stabilized with liquid for safer handling and transportation. If the substance is purchased and/or stored dry, then it falls under the controls in Work Process T, Chemicals with Explosive Properties. While sufficiently wetted or in solution, these materials can be handled without risk of detonation. However, if the liquid or solvent evaporates upon prolonged storage, the sensitive explosive material will be left behind and there is a risk of inadvertent detonation. The liquid used is sometimes quite volatile, such as diethyl ether or ethanol, and can evaporate quickly if containers are not properly resealed. Most reported incidents involving this class of material involve the friction-initiated detonation of dry crystals, either during scraping of the material as with a spatula, or when material has dried in the threads of the cap and the bottle is then opened.
- Time-Sensitive Gases
- Description of Hazard. This class of time-sensitive chemicals refers to pure gases or mixtures of gases that, due to their high reactivity, have the potential to over-pressurize, rupture, or corrode the cylinder in which they are provided. The valves on the cylinder may become corroded and blocked, causing the regulator to read zero pressure while there is still significant gas in the cylinder. If corrosion of valves or regulators is likely, a scale can be placed under the cylinder to keep track of its weight, rather than relying on the regulator pressure reading to determine how much gas is left in the cylinder.
In addition to the physical hazard of a cylinder failure, these gases are also highly toxic and/or reactive and present a major threat to life and health if they are released. In some cases, it may not be possible for someone entering the area after such an event to determine by sight or smell that the area is dangerous to enter. Further, gases have the potential to be released far outside the boundaries of a single room in which they are stored.
- Mixtures that include a low concentration (≤ 5%) of one of these gases in inert gas are not considered time-sensitive. Mixtures with more than 5% of one of these gases shall be assessed on a case by case basis using the professional judgement of the chemical owner and/or Designated Responsible Person, with consultation from EHS.
- It is especially important with these gases to be aware of all requirements and handling procedures for compressed toxic gas cylinders, as described in the ES&H Manual, Chapter 13, Gas Safety.
- Identification
- Gases that are known or reasonably suspected to have the capability to corrode or rupture their own cylinders upon prolonged storage are listed in Table 10.1.
- Return cylinders to the distributor or initiate the disposal process before the end of the cylinder’s shelf life.
- Storage
- Location: In general, containers of gases that are capable of rupturing their own cylinders are stored in an approved gas cabinet due to their toxicity and/or other hazards. Where not required to be stored in an approved gas cabinet, cylinders shall be seismically restrained and stored in accordance with ES&H Manual Chapter 13, Gas Safety.
- Condition: Store cylinders of these materials in a cool, dry location whenever possible. If cylinders are stored outside or in a wet or humid environment, ensure that all parts are fully dry before connecting a regulator and opening the cylinder. Water contamination can greatly increase corrosion rates.
- Assessment and Labeling
- This classification of time-sensitive chemical shall be monitored at least once every 6 months for the following failure criteria:
- Signs of corrosion or damage to the cylinder, especially near welding or valves, including frozen or stuck valves or blockages in the valve or regulator;
- Increased pressure inside the cylinder as read by a regulator (Note: if the cylinder is not connected to a regulator, do not connect it and open it simply to check);
- The expiration date/date of return as provided by the manufacturer has been met or exceeded or the cylinder has passed its default shelf life (see table above) and an expiration date or date of return was not provided by the manufacturer.
- Any time-sensitive material container that fails any one of these criteria shall be considered unsafe or expired, and the procedure in Section 11 shall be followed for the safe management and disposal of the container.
- If a corroded and blocked connection is suspected, close the valve on the cylinder and contact your Division Safety Coordinator and the Gases (Compressed/Hazardous/Toxic) subject matter expert for assistance.
- All containers of this class of time-sensitive chemical shall bear a label or hang-tag identifying them as a time-sensitive chemical. CMS and the label or tag must include the following information:
- A warning of potential for the gas to rupture its cylinder
- Date the cylinder was received
- Date the cylinder must be returned or disposal initiated
- This classification of time-sensitive chemical shall be monitored at least once every 6 months for the following failure criteria:
- Description of Hazard. This class of time-sensitive chemicals refers to pure gases or mixtures of gases that, due to their high reactivity, have the potential to over-pressurize, rupture, or corrode the cylinder in which they are provided. The valves on the cylinder may become corroded and blocked, causing the regulator to read zero pressure while there is still significant gas in the cylinder. If corrosion of valves or regulators is likely, a scale can be placed under the cylinder to keep track of its weight, rather than relying on the regulator pressure reading to determine how much gas is left in the cylinder.
- Management and Disposal of Unsafe, Expired or Unneeded Containers
- When any time-sensitive chemical, as covered by this policy, fails its assessment criteria (see Sections 7-10), exceeds its expiration date, or is no longer needed, the following steps shall be taken to ensure safe and compliant management of the container:
- If there is any reason to believe that the container poses an immediate danger, evacuate the immediate area and notify your Health and Safety Representative of the situation.
- If the container does not pose an immediate danger, contact your Health and Safety Representative and Waste Services Team Generator Assistant for evaluation of the container before taking any other actions. Do not store any time-sensitive materials in an SAA, WAA or MWSAA prior to evaluation by your Health and Safety Representative and Waste Services Team.
- Under the supervision of your Health and Safety Representative, remove any incompatible materials from the area of the time-sensitive chemical, if needed.
- Once your Health and Safety Representative and Generator Assistant have determined that the container is safe to handle, the Waste Services Team will determine the most appropriate plan for disposal.
- If it is determined that the container is not safe to handle, an independent contractor will be called in to develop a plan and remove the unsafe container from site.
- Only once instructed by the Waste Services Team to do so, follow the Waste Management Generator Guidelines, PUB-3092, for safe and compliant disposal of the container.
- When any time-sensitive chemical, as covered by this policy, fails its assessment criteria (see Sections 7-10), exceeds its expiration date, or is no longer needed, the following steps shall be taken to ensure safe and compliant management of the container:
- Lessons Learned
- Unstable/Reactive with Production of Gaseous Products
- Rust destabilized 4-nitrophenyl chloroformate:
http://www.crhf.org.uk/incident48.html - In 1943, pressure buildup in a bottle of stabilized ethyl chloroformate showered a stockroom worker at Cal Tech with a significant quantity of the chemical. The stockroom worker died at the hospital that night.
http://cenblog.org/the-safety-zone/2012/04/historical-accidents-at-caltech-via-linus-paulings-notebooks/
http://scarc.library.oregonstate.edu/coll/pauling/rnb/16/16-045.html
- Rust destabilized 4-nitrophenyl chloroformate:
- Hazardous Polymerization
- Thermal runaway of acrylic acid caused large release of liquid and vapor and subsequent fire:
http://www.crhf.org.uk/incident88.html
- Thermal runaway of acrylic acid caused large release of liquid and vapor and subsequent fire:
- Explosive When Dry
- A diazonium chloride salt precipitated from a supersaturated solution and detonated, killing the operator and injuring another:
http://www.crhf.org.uk/incident71.html - A postdoctoral researcher was injured while scraping residue of an explosive material from a filter paper. The explosive compound had been wetted during the procedure, but the residue had dried sufficiently that the scraping cased a detonation:
https://ehs.berkeley.edu/lesson-learned-dry-scraping-causes-chemical-explosion - While not directly caused by an explosive when dry compound, an incident involving sodium azide illustrates the severe consequences of contamination leading to more sensitive explosives. A low concentration sodium azide solution had been added to a particular piece of equipment to prevent algal growth in the water reservoir. A researcher removed a brass filter from the unit to clean it, and an explosion occurred during the cleaning procedure. While sodium azide is not sensitive to shock or friction, the copper azide formed on contact with the brass filter is a shock and friction sensitive explosive.
http://www2.lbl.gov/ehs/Lessons/pdf/LL_azidesv1.pdf
- A diazonium chloride salt precipitated from a supersaturated solution and detonated, killing the operator and injuring another:
- Time-Sensitive Gases
- An old lecture bottle of anhydrous hydrogen fluoride became over-pressurized and ruptured, causing extensive damage to a laboratory:
https://lessonslearned.lbl.gov/Open/preview.aspx?id=144 - Liquid hydrogen cyanide cylinders are known to undergo polymerization upon depletion of the added acid stabilizer. If heated, or if polymerized material from the wall of the cylinder falls into the liquid, the polymerization may proceed at an accelerating rate, causing a large explosion with release of unreacted HCN and other toxic gases:
http://osha.oregon.gov/OSHARules/interps/im-94-20.pdf
- An old lecture bottle of anhydrous hydrogen fluoride became over-pressurized and ruptured, causing extensive damage to a laboratory:
- Unstable/Reactive with Production of Gaseous Products
Work Process P.1 Tables
Table 7.1 Specific Chemicals with Pressure Hazards During Storage
| Specific Chemicals | CAS | Synonyms |
| Formic acid (≥ 98%) | 64-18-6 | Methanoic acid |
| Hydrogen Peroxide (≥ 30%) | 7722-84-1 | Dioxidane; Oxidanyl; Perhydroxic acid |
| Hydroxylamine | 7803-49-8 | Azinous acid; Aminol; Azanol; Hydroxyamine; Hydroxyazane; Hydroxylazane; Nitrinous acid |
| Borane-Tetrahydrofuran | 14044-65-6 | Borane-THF complex; Tetrahydrofuran compd. with borane |
Table 7.2 Chemical Categories with Pressure Hazards During Storage
| Chemical Categories | Examples |
| Chloroformates | Benzyl chloroformate (used for cbz protection); ethyl chloroformate; n-butyl chloroformate; sec-butyl chloroformate; isobutyl chloroformate; propargyl chloroformate; Fmoc chloride; nitrobenzyl chloroformate; nitrophenyl chloroformate; trichloroethyl chloroformate |
| Benzyl halides2 | Benzyl fluoride; Benzyl chloride; Benzyl bromide; Methylbenzyl chloride; Methylbenzyl bromide; Vinylbenzyl chloride; Methoxybenzyl chloride; Chloroxylene; α,α’-Dibromo-p-xylene; Methyl α-bromophenyl acetate |
| Halomethyl furans2 | 2-Chloromethylfuran; 2-Bromomethyl-5-methylfuran |
| Furoyl halides (Furan carbonyl halides) | 2-Furoyl chloride; 3-Methylfuran-2-carbonyl chloride |
| Halomethyl thiophenes2 | 2-Chloro-5-(chloromethyl)thiophene |
| Thiophene carbonyl halides | 2-Thiophenecarbonyl chloride |
| Benzene sulfinyl/sulfonyl halides | Benzene sulfinyl chloride; Benzene sulfonyl chloride |
2 Gas Evolution Incidents. Bretherick’s Handbook of Reactive Chemical Hazards, 6th Edition, pp 147-148.
3 Benzyl Compounds. Bretherick’s Handbook of Reactive Chemical Hazards, 6th Edition, pp 54-55.
2-Halomethyl-Furans or -Thiophenes. Bretherick’s Handbook of Reactive Chemical Hazards, 6th Edition, pp 167-168.





Work Process Q. Specific Controls and Procedures — Water-Reactive Chemicals
- General Information. Water-reactive chemicals react violently with water, releasing heat and, in some cases, explosive by-products. Of chief concern are GHS substances or mixtures which, in contact with water, emit flammable gases (Category 1: In contact with water, releases flammable gases which may ignite spontaneously). Examples of chemicals in this category include the alkali metals, sodium borohydride, lithium hydride, calcium hydride, phosphorus pentasulfide, borane complexes (borane-tetrahydrofuran, borane-methyl sulfide complex, etc.), and Grignard reagents. Some water-reactive chemicals in Category 1 are also pyrophoric, and must follow specific controls and procedures in Work Process R, in addition to those outlined in this Work Process.
The risks associated with water-reactive chemicals depend on the reactivity of the specific chemical, in addition to the hazards of gaseous products formed with water (flammable, toxic, or both).
Alkali metals react vigorously with water to formalkali
hydroxides and gaseous hydrogen. The alkali metal-water reaction is exothermic. The heat generated can ignite the hydrogen gas. The rate of reaction and the hazard severity increase as atomic weight increases. Lithium reacts slowest and poses the least hazard. Rubidium and cesium react explosively.
A DOE document, DOE-HDBK-1081-94, Primer on Spontaneous Heating and Pyrophoricity, provides additional information. - Control Measures
- Activity leads must identify water reactives used in the work area. Review sources such as SDSs for specific compounds.
- An assessment of the hazards and controls in place is necessary to safeguard employees against these agents. Contact an EHS Health and Safety Representative to provide assistance.
- Work involving these materials must be added to a Work Planning and Control Activity. Consult the Work Planning and Control program (ES&H Manual Chapter 6).
- Training and Information
- All employees who handle or may be exposed to water-reactive chemicals are required to complete Chemical Hygiene and Safety Training (EHS0348; or EHS0345 for Facilities personnel, Fire Extinguisher Safety (web-based) (EHS0520), and Fire Extinguisher Safety Retraining (EHS0531) annually thereafter. If desired, employees may also take Fire Extinguisher Safety (hands-on) (EHS0522).
- These individuals must be trained in the specific hazards and controls of the water-reactives. The Activity Lead and/or Project Lead is responsible for ensuring workers are properly trained, qualified, and authorized to work.
- Consult Work Process Y, Container Labeling, for labeling requirements for primary and secondary containers.
- The area entrance should be posted with a Caution Placard depicting the hazards and emergency contact information.
- Substitution and Chemical Inventory Management
- Identify and use safer chemical alternatives (e.g., non-water-reactive chemicals) if possible.
- If a safer chemical can’t be used, limit what you buy or borrow what you need from a colleague in your group or contact the Chemical Management System Coordinator (cms@lbl.gov) to assist you in finding a source of the chemical at Berkeley Lab.
- Conduct periodic clean-outs to prevent accumulating unneeded chemicals.
- Procure and use the minimum amount of material required for the operation, or
- Keep working quantities of chemicals to a minimum. Don’t stockpile chemicals.
- Enter these materials into the Chemical Management System (CMS).
- Engineering Controls. Alkali metals should be handled in a glove box made of materials that are compatible with the metal, with an inert gaseous atmosphere such as dry argon. Operations that could generate a flammable or toxic atmosphere must be performed in a fume hood.
- Work Practices
- Do not eat; drink; smoke; chew gum; apply cosmetics; or store food, beverages, and tobacco products in work areas where water-reactive materials are being used.
- General traffic should be prohibited in areas where alkali metal and water-reactive chemical operations are performed.
- Avoid all skin and eye contact with the material. Where possible, use tongs or appropriate tools to handle solids.
- All tools used to handle water-reactive chemicals including alkali metals must be dry, rust-free, clean, and composed of a compatible material. Tools can be dried by baking in an oven, desiccating in a vacuum, or rubbing with anhydrous dry soda ash.
- Protect water-reactive chemicals from water during use. Water may include atmospheric moisture. Air- and moisture-free handling techniques may be required.
- Develop procedures that will be used to mitigate the hazards of excess water-reactive chemicals and their mixtures after the experiment is completed. Quenching procedures must be authorized through work planning and control. Do not stockpile alkali metal “scrap” that is no longer needed, even if under mineral oil.
- Oxidized alkali metals (typically a white surface coating) make the material more hazardous to handle because the oxide can flake off. NOTE: Potassium metal with a yellow or orange coating may indicate the presence of peroxides, which may detonate if cut or abraded. Do not handle these materials. Contact an EHS Health and Safety Representative for further guidance. See Work Process P, Specific Controls and Procedures — Peroxide-Forming Compounds, for additional requirements.
- Assume that containers with water-reactive chemicals contain flammable (e.g. hydrogen) and/or toxic gas in the head space, even if stored under mineral oil or an inert gas. Thus, no source of ignition must be present where these containers are opened. Use non-sparking tools to open containers.
- Personal Protective Equipment (PPE). Skin and eye contact must be prevented. The following PPE will be worn when handling these materials. Additional information may be found in Work Process I, Personal Protective Equipment.
- At a minimum, safety glasses with side shields, laboratory coats (coveralls are acceptable in shop settings), and closed-toe shoes must be worn when handling these materials. For alkali metals, the shoes should be leather. This is to be considered as minimum protection and must be upgraded if necessary.
- Additional PPE such as chemical goggles, face shields, chemical aprons, fire-resistant aprons, disposable coveralls, chemically resistant gloves, and respiratory protection must be worn if there is a greater chance of chemical exposure. An EHS Health and Safety Representative may be contacted for assistance in selecting appropriate gloves and respiratory protection. The use of respiratory protection requires a hazard evaluation and a medical clearance followed by a fit test and training by the Research Support Team.
- Consult Work Process I.6, Eye and Face Protection, for guidance on the selection, uses, and limitations of safety glasses, chemical goggles, and face shields.
- Because many chemicals are skin-absorbers (i.e., agents that readily pass through the skin) it is important to select gloves that are chemically resistant to the material. Consult the PPE section, which contains a list of skin-absorbing agents and provides detailed guidance for selecting chemically resistant gloves.
- Gloves must be selected on the basis of their chemical resistance to the material(s) being handled, their suitability for the procedures being conducted, and their resistance to wear as well as temperature extremes. Improper selection may result in glove degradation, permeation of the chemical through the glove, and ultimately personal exposure to the chemical. This is a potentially serious situation. Consult Work Process I.5, Gloves, for guidance on the selection, uses, limitations, and disposal of chemically resistant gloves. An EHS Health and Safety Representative may also be contacted for assistance in selecting appropriate gloves.
- Storage
- Consult Work Process K, Chemical Storage, for hazardous material storage requirements, recommendations, and information on chemical incompatibility. It is recommended that alkali metals be stored in manufacturer-provided containers, if practical. Requirements for storing water-reactive chemicals are provided below.
- Separate alkali metals from incompatible chemicals. In addition to being water-reactive, alkali metals can react with oxygen, acids, halogenated hydrocarbons, and carbon dioxide.
- Store alkali metals under mineral oil or in an inert atmosphere.
NOTE: Lithium may react with nitrogen to form nitrides.
NOTE: Potassium may react with oxygen to form explosive peroxides. See Work Process P, Specific Controls and Procedures — Peroxide-Forming Compounds, for additional requirements.
- Emergency Procedures
- Consult Work Process V, Emergency Procedures and Equipment, for emergency actions regarding chemical spills and personal exposure to chemicals.
- Employees working with water-reactive chemicals must take Fire Extinguisher Safety (web-based) (EHS0520), and Fire Extinguisher Safety Retraining (EHS0531) annually thereafter. If desired, employees may also take Fire Extinguisher Safety (hands-on) (EHS0522).
- In addition to these requirements, the following applies to spills of water-reactive compounds:
- Never use combustible or reactive materials (such as paper towels) to clean up spills. Keep an adequate number of appropriate spill kits to meet anticipated needs.
- In case of a fire, the safest course of action is to notify the fire department by activating a fire alarm pull station, call x7911 or 911, and evacuate the building. Employees who are comfortable doing so may attempt to extinguish a small fire with a fire extinguisher only after activating a fire alarm pull station, and only if the employee knows what is burning to select the appropriate extinguisher, can fight the fire without sustaining injury, and fighting the fire will not prevent the employee’s escape from the building if the fire is not contained.
- Anhydrous dry soda ash may be used for fires involving all metals except lithium. Lith-X fire extinguishers must be used for lithium metal fires. Met-L-X metal fire extinguishers may be used for sodium, potassium, and sodium-potassium alloy (NaK) fires. Employees involved in metal work must be knowledgeable of the details of the emergency plan in case of a metal fire, e.g. the nearest pull station, evacuation routes, location of fire extinguishers, etc.
Please note that Met-L-X, Lith-X, and other Class D fire extinguishers are not suitable for flammable organic solvent fires, even if the fire was initiated by reaction with an alkali metal or water-reactive chemical.
- Skin or Eye Contact
- If any alkali metal fragment or drop enters the eye, it will immediately generate considerable heat, which is likely to result in severe eye injury. In such cases, the eyes should be flushed with water from an eyewash/safety shower. Continue to flush the eye with water while someone dials 7-911 for emergency help.
- When alkali metal comes in contact with the skin, remove all contaminated clothing. If contact with the metal occurs at only one or two spots on the skin, it is best to wash off those areas with mineral oil. A container with at least one quart of mineral oil should be available in alkali metal work areas labeled for this purpose. If contact with the metal is widely distributed over the body, a decision on the best course of first aid must be made immediately. If the material is already burning, the individual should be drenched continually under a safety shower until emergency help arrives. If the material is not burning, the metal should be removed by wiping the skin with mineral oil. In all cases, dial 7-911 for assistance.
- Disposal
- Contact the Waste Management Group Generator Assistant if you need guidance or assistance on proper disposal of water-reactive chemicals. Water-reactive chemicals must be packaged in a compatible container and meet off-site vendor’s disposal waste acceptance criteria.
- Contact your Division’s Generator Assistant to ensure that your waste is safe for pickup, storage, and transportation and meets waste disposal waste acceptance criteria.
- Alkali metals (e.g., lithium, potassium, rubidium, and cesium) must be fully submerged in mineral oil before placement in a SAA or WAA.
- Water-reactive non-alkali metal waste must be packaged in a compatible container under inert conditions (e.g., under argon or nitrogen) before placement in a SAA or WAA.
- Contact your Division’s Generator Assistant to assist with unique water-reactive waste before generating the waste (e.g., experimental lithium batteries or more than 500 grams of alkali metals).
Work Process R. Specific Controls and Procedures — Pyrophoric Materials
- General Information
- Pyrophoric materials ignite spontaneously when exposed to air. Moreover, they are commonly associated with flammable solvents such as pentane, hexane, heptane, and diethyl ether. This combination poses a significant hazard to users. Other hazards posed by these materials include corrosivity, water reactivity, peroxide formation, and toxicity.
- A number of common reagents are pyrophoric, including (but not limited to):
- Organolithium reagents. Typically in hydrocarbon solvents. Note: Tert-butyllithium solutions are highly pyrophoric.
- Organomagnesium reagents. These include Grignard reagents (RMgX). Typically in hydrocarbon solvents. Neat reagents are pyrophoric.
- Organoaluminum reagents. Neat or in hydrocarbon solvents. Neat reagents are highly pyrophoric.
- Organozinc reagents. Neat reagents are pyrophoric.
- Boranes. Neat reagents are pyrophoric.
- Other pyrophoric liquids include metal alkyls such as trimethylaluminium, trimethylgalium, and trimethylindium.
- It is imperative that personnel understand the hazards associated with pyrophoric materials and their solvents, and understand how to control these hazards.
- Substitution and Chemical Inventory Management
- Prior to purchasing a pyrophoric material, users shall:
- Review the hazards of the material and assess the conditions under which it will be used. Hazard information may be obtained from the SDS or other information sources such as Bretherick’s Handbook of Chemical Reactive Hazards or by consulting with EHS Health and Safety Representatives.
- Identify and use safer chemical alternatives if possible.
- Limit the amount purchased. As an alternative, consider borrowing what you need from a colleague in your group or contact the Chemical Management System Coordinator (cms@lbl.gov) to assist you in finding a source of the chemical at Berkeley Lab.
- Enter pyrophoric materials into the Chemical Management System (CMS).
- Keep working quantities to a minimum. Store and use the minimum for the operation. Then dispose of the excess.
- Not stockpile pyrophoric chemicals.
- Conduct periodic cleanouts to prevent accumulating unneeded pyrophoric chemicals.
- Prior to purchasing a pyrophoric material, users shall:
- Work Planning and Control. Work involving these materials shall be added to a Work Planning and Control Activity. Consult the Work Planning and Control program (EH&S Manual Chapter 6).
- Training and Qualifications
- Chemical Hygiene and Safety Training (EHS0348) and Fire Extinguisher Safety (EHS0520 and EHS0522) are required. However, because technique and handling practices are critical, on-the-job training (OJT) given by a knowledgeable, experienced worker, such as the Work Planning and Control Activity Lead shall be the primary training method. OJT must be documented in the WPC Activity. The objectives of OJT are to ensure that users:
- Understand the hazards of pyrophorics
- Understand the controls for pyrophorics
- Know, understand and use:
- Safe storage practices
- Labeling procedures. Consult Work Process Y, Container Labeling, for labeling requirements for primary and secondary containers.
- Safe handling practices, including transferring and use of equipment and apparatuses such as syringes and Schlenk lines
- Engineering controls
- Selection and use of PPE
- Users may work with pyrophoric materials unsupervised, provided that all of the following conditions are met:
- OJT is completed and documented.
- The activity lead has observed the user performing an unassisted procedure.
- The user has demonstrated proficiency in the safe handling and use of pyrophorics to the satisfaction of the activity lead.
- Both the user and the activity lead are confident that the user can perform the work safely.
- Chemical Hygiene and Safety Training (EHS0348) and Fire Extinguisher Safety (EHS0520 and EHS0522) are required. However, because technique and handling practices are critical, on-the-job training (OJT) given by a knowledgeable, experienced worker, such as the Work Planning and Control Activity Lead shall be the primary training method. OJT must be documented in the WPC Activity. The objectives of OJT are to ensure that users:
- Safe Storage Methods
- Consult Work Process K, Chemical Storage, for hazardous material storage requirements, recommendations, and information on chemical incompatibility. Additional requirements are provided below.
- Store pyrophorics in an inert glove box. Pyrophoric materials and flammable liquids may be stored in the same inert glove box.
- If storing in an inert glove box is not possible, keep pyrophorics in an airtight container and store in a flammable-storage locker specifically designated for pyrophorics. Pyrophoric materials and flammable liquids may not be stored together in the same flammable storage locker.
- Keep pyrophorics in their original containers (e.g., Sure/Seal bottles) unless experimental work requires transfer to other containers such as Straus flasks. Note: Sure/Seal bottles may leak when the septum is perforated. Therefore, inspect them on a regular basis and replace caps in an inert glove box if necessary.
- Pyrophoric materials may be stored in refrigerators designed and constructed for storing flammable liquids. However, the refrigerator(s) must be specifically designated for pyrophorics. Pyrophoric materials and flammable liquids may not be stored together in the same refrigerator. Consult the Work Process K, Chemical Storage, for additional requirements for refrigerators.
- Use secondary containment for all liquids.
- Handling, Transfer, and Use of Pyrophorics. All equipment in a pyrophoric reaction must be used following air-sensitive techniques:
- Oven-dry glassware used for pyrophorics.
- Flush glassware, syringes, and conveyance lines with argon or nitrogen.
- Use syringes equipped with Luer Locks to secure needles.
- Limit maximum volume that can be transferred with a syringe to 20 ml.
- Use cannulas for transferring larger volumes of pyrophorics.
- Clamp the reagent bottle and the receiving vessel to prevent tipping and to allow the free use of both hands.
- Conduct operations inside an inert glove box when possible.
- When it is not practical to use a glove box, conduct operations in a fume hood.
- Keep the sash of the hood to the lowest practical height.
- Glove boxes and fume hoods must have a current approval sticker.
- Keep flammable and combustible loading to a minimum in fume hoods and glove boxes. This includes reagents, paper, and cloth.
- More detailed descriptions may be found in The Manipulation of Air-Sensitive Compounds (Shriver and Drezdzon; John Wiley & Sons, New York, 1986), and in Aldrich Technical Bulletin AL-134, Handling Air-Sensitive Reagents, which is included with the purchase of air-sensitive chemicals and may be obtained from the Aldrich Technical Bulletins Web site.
- PPE and the Use of Nomex (fire-retardant) Lab Coats
- The minimum PPE for handling pyrophorics outside of inert glove boxes is: Nomex lab coat (or equivalent), safety glasses with side shields, long pants, closed toed shoes, and chemically resistant gloves. Disposable lab coats and lab coats made of polyester blends are prohibited for use with pyrophoric materials. If Nomex lab coats are not practical, a fire-retardant cotton lab coat may be used provided that a laundry is employed that is approved for laundering such garments in accordance with the manufacturer’s requirements. Lab coats may be ordered and laundered through a service such as Mission Linen Supply.
- Cover goggles and/or face shields must be used as warranted by the hazard. Note: Face shields must be worn in conjunction with approved safety glasses or cover goggles. Consult Work Process I.6, Eye and Face Protection, or an EHS Health and Safety Representative for further guidance.
- Glove selection will normally be based on the solvent containing the pyrophoric material. Consult Work Process I.5, Gloves, or an EHS Health and Safety Representative for further guidance.
- Emergency Procedures, including Extinguishing Media for Fires
- Consult Work Process V, Emergency Procedures and Equipment, for emergency actions regarding chemical spills and personal exposure to chemicals.
- In addition to these requirements, the following applies to spills and fires involving pyrophoric compounds:
- Safety must be the primary concern regarding spills. Do not attempt to clean up pyrophoric material spills that occur in an ambient atmosphere. Warn others, leave the area, and call the emergency number on the nearest safe lab phone (7-911 at Berkeley Lab).
- You may clean up spills in inert glove boxes only if it can be done safely.
- Avoid using combustible or reactive materials (such as paper towels) to clean up spills.
- Keep material on hand to absorb spills. Inorganic diatomaceous earth (Celite), clay-based kitty litter, and/or molecular sieves (13X) may be used. Ensure these materials are dry.
- Appropriate Class D fire extinguishers must be staged outside the work area. There are several different types of extinguishing media. Contact Berkeley Lab’s Fire Marshal for proper selection.
- If researchers choose to stage Class D fire extinguishers in the laboratory area, they will be responsible for performing the monthly inspections and for coordinating fire extinguisher service with Laboratory’s contractor. Contact Berkeley Lab’s Fire Marshal for guidance on selecting, staging, and inspecting fire extinguishers and for coordinating fire extinguisher services.
- If you use a fire extinguisher, ensure you empty the entire contents. As an option, plastic baggies of fire-extinguishing agent, such as Met-L-X or Lith-X, may be kept in inert glove boxes where the agent is appropriate for the material being handled. These baggies are not a substitute for the appropriate fire extinguisher but rather serve as a handy material to help extinguish a small fire. Employees must have OJT in the proper use of these extinguishing materials.
- Quench or otherwise neutralize spill clean-up materials prior to removing from the inert glove box.
- Only trained personnel may attempt to control small, contained fires or spills. If fumes escape into the breathing zone, do not attempt to put out the fire. Large or unconfined fires or spills, or fires where the ventilation system (glove box or fume hood) does not contain all of the fumes, must be handled by fire fighters. When in doubt, activate the nearest fire alarm and call the emergency number on the nearest safe lab phone (7-911 at Berkeley Lab).
- Disposal
Information on waste disposal may be found in Guidelines for Generators to Meet HWHF Acceptance Requirements for Hazardous, Radioactive, and Mixed Wastes at Berkeley Lab Waste Generator Guidelines (PUB-3092). Only those who have completed Hazardous Waste Generator Training (EHS0604) or Radioactive/Mixed Waste Generator Training (EHS0622) are permitted to add waste to a Satellite Accumulation Area (SAA) or Mixed Waste Satellite Accumulation Area (MWSAA) or Waste Accumulation Area (WAA).
- Contact your Division’s Generator Assistant for guidance or assistance on proper disposal of pyrophoric materials to ensure that your waste is safe for pickup, storage, and transportation and waste disposal waste acceptance criteria.
- Pyrophoric chemicals must be packaged in a compatible container under inert conditions (e.g., under argon or nitrogen) before placement in a SAA or WAA to ensure it meets off-site vendor’s waste disposal waste acceptance criteria.
Work Process R.1 Specific Controls and Procedures — Chemical Synthesis
- General Information
- The focus of this Work Process R.1 is on the use of air- and water-free techniques.
- The hazards associated with chemical synthesis involve the use of chemical agents, including water-reactive and pyrophoric agents, and physical hazards related to the use of apparatuses such as cold traps and vacuum lines.
- The hazards and controls of water-reactive and pyrophoric agents are discussed in detail in Work Process Q and Work Process R, respectively.
- Integrated Safety Management (ISM) for Planning Chemical Synthesis
Workers must perform
ISM daily before the start of an experiment to:
- Determine if any changes alter the Scope of Work to an extent that would require further evaluation of the hazards, including:
- The need to go beyond previously established limits such as reaction scale, volume, weight, or concentration, temperature, pressure, time, velocity, etc.
- Introduction of new chemical(s), especially chemical hazards not included in work planning or chemical substitution.
- Equipment changes.
- Procedure changes, including waste/clean-up/quenching steps.
- Location of the work changes.
- Determine if any changes alter the Scope of Work to an extent that would require further evaluation of the hazards, including:
Workers must also:
- Plan the synthesis in advance of starting any chemical procedure.
- Outline the expected reaction and determine any expected/potential side reactions that may be involved.
- Read and understand relevant articles on the synthesis to be performed (or on similar syntheses if the procedure is new) in order to gain insight into how the reaction should behave.
- Determine experimental conditions (i.e., the use of Schlenk lines, high-pressure reactors, heating methods and reaction temperatures, flask sizes, reaction times, syringe or cannula sizes for transfer, etc.).
- Design experiments to be as low a hazard level as possible.
- Select starting materials that have the lowest hazard level possible, or that form less hazardous side products during the synthesis.
- Be mindful of hazardous reaction conditions and select the safest method.
- Establish a reaction clean-up procedure for the product and reaction vessels. Establish a procedure for disposing of any reaction by-products and washing media, and other wastes. Identify all anticipated waste streams, and establish labeling and storage requirements for each. Include steps for minimizing the quantity and/or hazards of waste to be generated.
- If the synthesis is unfamiliar to the researcher, consult with more experienced researchers while developing the reaction. This can be facilitated by contacting the Division Safety Coordinator (DSC) or the EHS Division Liaison.
- Discuss the reaction plan and hazards with the Activity Lead, Project Lead, or supervisor as appropriate, and consider what might go wrong at any step with the reaction or the experimental apparatus.
- Ensure that all work is authorized under a relevant Work Planning & Control (WPC) Activity.
- If there is a significant change in the scope of work, or if additional WPC Risk Level 3 hazards are added to the Activity, then a new version of the Activity must be started and the Activity must go through the collaboration and approval processes.
- Define the Scope of Work for the reaction, and have the procedure approved by the Activity Lead.
- Clearly establish the allowed reaction conditions. Examples include establishing an allowed reaction scale range, suitable reagent substitutes (especially reducing and oxidizing agents), reaction temperatures and times, etc.
- Clearly establish triggers that will require re-evaluation. When a researcher wishes to go beyond the original Scope of Work that was previously defined and approved, the desired change and any potential new hazards must be discussed with the supervisor and approved prior to continuing with the work.
- Analyze hazards.
- Read and understand the Safety Data Sheets (SDSs) of all reagents and anticipated side-reaction products that may be involved.
- Be mindful of hazards that may be generated as the reaction progresses (e.g., Is there an increase in pressure? Will any side products react unfavorably? Will pyrophoric products form? etc.).
- Be aware of the hazards inherent to the reaction set-up (e.g., solvent traps in nitrogen may form liquid oxygen if air is introduced, glassware may fail, temperature controllers may overheat,
stirring may be inadequate or fail,
etc.).
Be aware of the hazards of large-scale reactions (e.g. greater than 500mL of flammable liquids or greater than 1L glass reaction vessel). Analyze for inefficiencies in mixing, heating, or cooling and surface-area-to-volume ratios as scale increases.
- Researchers without the expertise to perform an accurate hazard analysis must discuss the potential hazards with someone who is more experienced than they.
- If you have any doubt or are unfamiliar with any aspect of the experiment, seek out the help of a more experienced person to assist with a thorough hazard analysis.
- Potential hazards must be discussed with and understood by the supervisor prior to starting work.
- Develop a hazard plan, and implement controls.
- Know the potential hazards of the reaction by consulting SDSs and reading literature on how the chemicals involved may interact.
- Plan the reaction to prevent known hazards (e.g., if the introduction of air will cause fire, then perform the reaction under argon; when using a pressure reactor, calculate potential pressure build up of the reaction, and design the reaction to stay well below the pressure threshold of the reactor; etc.).
- Have a plan in place to deal with hazards that were not originally foreseen or were not prevented (e.g., know where the nearest spill kits, fire alarm pull station, evacuation path, Emergency Assembly Area, and fire extinguisher are located and how to use them; know how to quickly quench problematic reactions; know when to call for help; etc.).
- Determine the appropriate types of personal protective equipment (PPE) necessary for the reaction. PPE may include closed-toe shoes, lab coat, safety glasses, blast shield, face shield, special gloves, etc.
- Ensure that all appropriate on-the-job (OJT) training has been performed, and that the researcher has been approved on any WPC activities required for the work. Inexperienced researchers may require additional supervision and approval to work independently.
- Determine how the Work Alone policy will apply to the reaction.
- Ensure the hazard plan addresses safe handling and controls for waste chemicals that will be generated in the reaction.
- Perform work. After the reaction and its hazards are understood and the scope of work has been approved by the supervisor, the reaction may be performed.
- Get feedback and improve. Analyze the reaction after it has been performed, paying special attention not only to the product but also to the overall method and any unexpected hazards. Evaluate how the reaction went, and use what was learned to plan the next reaction.
- Review existing procedures, hazards, and controls
- Plan the synthesis in advance of starting any chemical procedure.
- Outline the expected reaction and determine any expected/potential side reactions that may be involved.
- Read and understand relevant articles on the synthesis to be performed (or on similar syntheses if the procedure is new) in order to gain insight into how the reaction should behave.
- Determine experimental conditions (i.e., the use of Schlenk lines, high-pressure reactors, heating methods and reaction temperatures, flask sizes, reaction times, syringe or cannula sizes for transfer, etc.).
- Design experiments to be as low a hazard level as possible.
- Select starting materials that have the lowest hazard level possible, or that form less hazardous side products during the synthesis.
- Be mindful of hazardous reaction conditions and select the safest method.
- Establish a reaction clean-up procedure for the product and reaction vessels. Establish a procedure for disposing of any reaction by-products and washing media, and other wastes. Identify all anticipated waste streams, and establish labeling and storage requirements for each. Include steps for minimizing the quantity and/or hazards of waste to be generated.
- If the synthesis is unfamiliar to the researcher, consult with more experienced researchers while developing the reaction. This can be facilitated by contacting the Division Safety Coordinator (DSC) or the EHS Division Liaison.
- Discuss the reaction plan and hazards with the Activity Lead, Project Lead, or supervisor as appropriate, and consider what might go wrong at any step with the reaction or the experimental apparatus.
- Ensure that all work is authorized under a relevant Work Planning & Control (WPC) Activity.
- If there is a significant change in the scope of work, or if additional WPC Risk Level 3 hazards are added to the Activity, then a new version of the Activity must be started and the Activity must go through the collaboration and approval processes.
- Define the Scope of Work for the reaction, and have the procedure approved by the Activity Lead.
- Clearly establish the allowed reaction conditions. Examples include establishing an allowed reaction scale range, suitable reagent substitutes (especially reducing and oxidizing agents), reaction temperatures and times, etc.
- Clearly establish triggers that will require re-evaluation. When a researcher wishes to go beyond the original Scope of Work that was previously defined and approved, the desired change and any potential new hazards must be discussed with the supervisor and approved prior to continuing with the work.
- Analyze hazards.
- Read and understand the Safety Data Sheets (SDSs) of all reagents and anticipated side-reaction products that may be involved.
- Be mindful of hazards that may be generated as the reaction progresses (e.g., Is there an increase in pressure? Will any side products react unfavorably? Will pyrophoric products form? etc.).
- Be aware of the hazards inherent to the reaction set-up (e.g., solvent traps in nitrogen may form liquid oxygen if air is introduced, glassware may fail, temperature controllers may overheat, stirring may be inadequate or fail, etc.).
- Be aware of the hazards of large-scale reactions (e.g. greater than 500mL of flammable liquids or greater than 1L glass reaction vessel). Analyze for inefficiencies in mixing, heating, or cooling and surface-area-to-volume ratios as scale increases.
- Researchers without the expertise to perform an accurate hazard analysis must discuss the potential hazards with someone who is more experienced than they.
- If you have any doubt or are unfamiliar with any aspect of the experiment, seek out the help of a more experienced person to assist with a thorough hazard analysis.
- Potential hazards must be discussed with and understood by the supervisor prior to starting work.
- Develop a hazard plan, and implement controls.
- Know the potential hazards of the reaction by consulting SDSs and reading literature on how the chemicals involved may interact.
- Plan the reaction to prevent known hazards (e.g., if the introduction of air will cause fire, then perform the reaction under argon; when using a pressure reactor, calculate potential pressure build up of the reaction, and design the reaction to stay well below the pressure threshold of the reactor; etc.).
- Have a plan in place to deal with hazards that were not originally foreseen or were not prevented (e.g., know where the nearest spill kits, fire alarm pull station, evacuation path, Emergency Assembly Area, and fire extinguisher are located and how to use them; know how to quickly quench problematic reactions; know when to call for help; etc.).
- Determine the appropriate types of personal protective equipment (PPE) necessary for the reaction. PPE may include closed-toe shoes, lab coat, safety glasses, blast shield, face shield, special gloves, etc.
- Ensure that all appropriate on-the-job (OJT) training has been performed, and that the researcher has been approved on any WPC activities required for the work. Inexperienced researchers may require additional supervision and approval to work independently.
- Determine how the Work Alone policy will apply to the reaction.
- Ensure the hazard plan addresses safe handling and controls for waste chemicals that will be generated in the reaction.
- Perform work. After the reaction and its hazards are understood and the scope of work has been approved by the supervisor, the reaction may be performed.
- Get feedback and improve. Analyze the reaction after it has been performed, paying special attention not only to the product but also to the overall method and any unexpected hazards. Evaluate how the reaction went, and use what was learned to plan the next reaction.
- Plan the synthesis in advance of starting any chemical procedure.
- Work Planning and Control
- Chemical synthesis hazards and controls must be described and documented in the WPC system.
- Each activity must be planned with a well-defined scope and proper hazard identification.
- New or additional hazards may be introduced into activities where changes in the scope of work, scale, or other conditions occur. Therefore, trigger points (i.e., agreed-upon thresholds above the established scopes of work) must be established for each synthesis activity to prompt hazard reviews and additional OJT when these changes occur.
- It is equally important that workers communicate changes in their work to activity leads and safety line management as appropriate in order to ensure that the hazards associated with these changes are identified and controlled.
- Scale-up and large-scale chemical synthesis activities require a higher level of review and approval prior to the start of work (Risk Level 3 – High Risk).
- Examples of high-risk chemical synthesis work include, but are not limited to:
- Use of flammable and combustible liquids in containers, such as glass round-bottom flasks, above maximum sizes designated for material type per Ch. 12, Fire Prevention and Protection, Appendix A.
- Chemical synthesis tasks at greater than 500mL solvent or 1L or greater flask size involving heterogeneous, biphasic, or polyphasic reaction mixtures or highly exothermic reactions. Quenching, purification, and work-up steps involving these conditions must also be evaluated.
- Reactions that generate gaseous by-products (i.e. off-gas) via fast decomposition.
- Resources for identifying high-risk scale-up and large-scale synthesis are available, including chemical hazard-specific guidance, relevant Lessons Learned, and template WPC activities.
- Examples of high-risk chemical synthesis work include, but are not limited to:
- Chemical Inventory Management
- Consult Work Process B, Chemical and Equipment Procurement, for institutional expectations that must be met prior to purchasing chemicals and equipment associated with chemical synthesis.
- Consult Work Process D, Berkeley Lab Chemical Inventory, for chemical inventory management requirements.
- Training and Qualifications
- Employees who perform chemical synthesis must complete Chemical Hygiene and Safety Training (EHS0348), Fire Extinguisher Safety (web-based) (EHS0520), and Fire Extinguisher Safety Retraining (EHS0531) annually thereafter. If desired, employees may also take Fire Extinguisher Safety (hands-on) (EHS0522). Additionally, Fire Extinguisher Safety (hands-on) (EHS0522) is required for employees working with pyrophoric materials. See Work Process R.
- Activity leads who provide oversight for chemical synthesis activities must be qualified to identify hazards, establish controls, and authorize workers to perform the activities. This is also needed to provide effective OJT.
- OJT must have sufficient specificity and detail for workers to understand the hazards and controls of their activities so they can be performed safely.
- OJT shall be considered to be complete when:
- The user has demonstrated unassisted proficiency in the safe handling and use of the chemicals and apparatus to the satisfaction of the Activity Lead.
- Both the user and the activity lead are confident that the user can perform the work safely.
- The OJT given has been described and documented (via WPC “Authorization to Work Unsupervised”) in the WPC Activity.
- Safe Storage Methods
- Consult Work Process K, Chemical Storage, for hazardous material storage requirements, recommendations, and information on chemical incompatibility. Additional requirements are provided below.
- Materials and reagents used in chemical synthesis include pyrophorics, water reactives, reducing and oxidizing agents, air reactives, etc. Generally, these materials are kept in inert environments such as glove boxes. Refer to Work Process Q for the storage requirements of water-reactive chemicals, and to Work Process R for the storage requirements of pyrophoric materials.
- Ensure all excess and unwanted chemicals to be disposed of as waste are stored safely. Chemicals that pose a health and safety hazard cannot be stored in a Satellite Accumulation Area (SAA) or a Waste Accumulation Area (WAA) without first mitigating the hazards. Refer to Work Process Q for the storage requirements of water-reactive chemicals, and to Work Process R for the storage requirements of pyrophoric materials.
- Waste Disposal
- Handle, label, and store all waste properly. Consult with your division’s EHS Waste Generator Assistant for guidance on the proper handling and disposal of chemicals used in the lab for chemical synthesis.
- Additional information on waste disposal may be found in the Waste Management program (ES&H Manual, Chapter 20) and in Guidelines for Generators to Meet HWHF Acceptance Requirements for Hazardous, Radioactive, and Mixed Wastes at Berkeley Lab Waste Generator Guidelines (PUB-3092).
- Only those who have completed Hazardous Waste Generator Training (EHS0604) are permitted to add waste to an SAA.
Work Process S. Specific Controls and Procedures — Engineered Nanomaterials
- General Information
- Engineered nanomaterials (ENMs), also known as engineered nanoparticles, are defined as:
- Materials having structures with at least one dimension between 1 and 100 nanometers (nm)
- Intentionally created, as opposed to those that are naturally or incidentally formed
- ENMs do not include:
- Larger materials that may have nanoscale features, for example etched silicon wafers
- Biomolecules (proteins, nucleic acids, and carbohydrates)
- Materials with occupational exposure limits (OELs) that address nanosize particles for that substance
- Unbound engineered nanoscale particles (UNPs) are defined as nanoscale particles that are not contained within a matrix under normal temperature and pressure conditions that would reasonably be expected to prevent the particles from being separately mobile and a potential source of exposure.
- An engineered primary nanoscale particle dispersed and fixed within a polymer matrix, incapable as a practical matter of becoming airborne, would be “bound,” while such a particle loosely attached to a surface (e.g., nanowire forest grown on wafer) or suspended in liquid (e.g., nanoparticles in colloidal suspension) or a dry powder would be “unbound.”
- A UNP worker is a worker who:
- Has the potential for inhalation of or dermal exposure to UNPs
- Routinely spends time in an area in which engineered UNPs have the potential to become dispersed in the air or on surfaces or
- Works on equipment that might contain or bear UNPs and that could release UNPs during servicing or maintenance
- Exposures to ENMs may occur through inhalation, dermal contact, or ingestion of UNPs. Because of their tiny size, UNPs can penetrate deep into the lungs and may translocate to other organs following pathways not demonstrated in studies with larger particles.
- In general, laboratory personnel should treat all new compounds, including ENMs of unknown toxicity, as though they could be acutely toxic in the short run and chronically toxic over time. ENMs whose hazards have been studied should be managed in a manner consistent with the observed risks.
- Work involving these materials shall be added to a Work Planning and Control Activity. Consult the Work Planning and Control program (EH&S Manual Chapter 6).
- Engineered nanomaterials (ENMs), also known as engineered nanoparticles, are defined as:
- Training and Information
- Employees who either handle or who may be exposed to the hazards of ENMs must complete Chemical Hygiene and Safety Training (EHS 348) and Safe Handling of Engineered Nanoscale Particulate Matter (EHS 344).
- Activity leads are responsible for on-the-job training specific to hazards and controls of these materials for their work activities. Information on hazards and minimum PPE requirements must be available to workers accessing work areas where these hazards are present such as through the entrance placard and co-located hazards in WPC activities. EHS Health and Safety Representatives are available to provide assistance.
- Engineering Controls
- Conduct work that could generate UNPs in glove boxes, glove bags, laboratory fume hoods, or other negative-pressure or isolation enclosures. If a process (or subset of a process) cannot be enclosed, use other engineered systems to control fugitive emissions of UNPs or hazardous precursors that might be released. For example, use a local exhaust system such as an extractor arm.
- With regard to gloves worn in glove boxes: Consider using an inner pair of different-color gloves to detect small tears in glove-box gloves and/or wearing an outer pair of gloves to prevent degradation of glove-box gloves. Refer to Work Process I, Personal Protective Equipment, for selecting chemically resistant gloves.
- Avoid exhausting effluent air reasonably suspected to contain UNPs whose hazards are not well understood. Whenever practical, filter it or otherwise clean (scrub) it before release. High Efficiency Particulate Arresting (HEPA) filtration appears to effectively remove UNPs from air.
- Do not use horizontal laminar-flow hoods (“clean benches”) that direct a flow of air into the laboratory to control exposure to UNPs.
- Consider exhausting Type II biological safety cabinets, in which free UNPs are handled, directly to the exterior (hard-ducted) or through a thimble connection over the cabinet’s exhaust.
- Evaluate laboratory equipment and exhaust systems for contamination before removing, remodeling, or repairing them.
- Housekeeping
- In so far as practicable, maintain all working surfaces (e.g., benches, glassware, apparatus, exhaust hoods, support equipment) free of UNP contamination. Some UNPs fluoresce under ultraviolet light, which can be useful in locating areas of contamination.
- Clean up dry UNPs using:
- A dedicated HEPA vacuum-tested and certified by EHS
- Wet wiping
- Other methods that do not involve dry sweeping or the use of compressed air
- Dispose of used cleaning materials and wastes as hazardous waste (see below).
- Chemical Inventory
- Enter all containers of commercially obtained ENMs into the Chemical Management System (CMS). The CMS has a checkbox for ENMs to allow easier identification of storage and use locations. Ensure this is checked when entering the material into the inventory.
- Marking, Labeling, and Signage
- Post signs indicating hazards, minimum PPE requirements, and administrative control requirements at entry points into areas where ENMs are handled. Work Process AA, Posting Area Entrances, has specific posting requirements and instructions.
- Consult Work Process Y, Container Labeling, for labeling requirements for primary and secondary containers. Label containers to plainly indicate that the contents are in engineered nanoparticulate form, e.g., “nanoscale zinc oxide particles” or other identifier, rather than just “zinc oxide.”
- There may be practical limitations to carrying out these labeling requirements to small containers such as sample vials and tubes. Alternatives such as numbering or coding are permissible provided that the material’s identity and hazards are readily accessible (e.g., by means of a lab notebook, a spreadsheet, or some other equivalent means).
- When UNPs are being moved outside the work area, also include label text that indicates that the particulates may be unusually reactive and are potentially more toxic, quantitatively and qualitatively, than normal-scale forms of the same material.
- Storage
- Consult Work Process K, Chemical Storage, for hazardous-materials storage requirements, recommendations, and information on chemical incompatibility. Additional requirements are provided below.
- Follow the storage guidelines in Work Process N, Specific Controls and Procedures — Flammables and Combustibles if the material is either flammable or combustible.
- Personal Protective Equipment
- Skin and eye contact must be prevented. Wear PPE appropriate to the hazard, as identified through the WPC Activity. Obtain a hazard assessment from an EHS Health and Safety Representative to determine the selection and use of PPE. PPE required for a wet-chemistry laboratory, which is often appropriate for handling ENMs, includes:
- Laboratory coats
- Eye protection, e.g., safety glasses with side shields, face shields, chemical splash goggle, or other safety eyewear appropriate to the type and level of hazard. NOTE: Face shields or safety glasses alone do not provide sufficient protection against unbound, dry materials that could become airborne.
- Closed-toe shoes made of a low-permeability material
- Protective gloves
- Store gloves in a clean area outside of fume hoods and away from equipment that could potentially contaminate them.
- Wear polymer (e.g., nitrile rubber) gloves when handling ENMs and particulates in liquids. Choose gloves only after considering the resistance of the glove to the chemical attack both by the ENM and, if suspended or dissolved in liquid, the liquid. Consult the glove selection guides in Work Process I, Personal Protective Equipment. Disposable gloves may be appropriate.
- Change gloves often to minimize potential exposure hazards. Alternatively, double-glove.
- For glove-box gloves: Consider using an inner pair of different-color gloves to detect small tears in glove-box gloves and/or wearing an outer pair of gloves to prevent degradation of glove-box gloves.
- Wash hands and forearms after wearing gloves.
- Keep potentially contaminated clothing and PPE in the laboratory or change-out area to prevent ENMs from being transported into common areas. Use disposable lab coats if feasible, and discard of them as hazardous waste (see below) when they become unusable. If cloth lab coats are used, do not send them to a laundry unless the laundry (such as the Berkeley Lab contract lab coat provider) has specifically agreed to handle ENM-contaminated clothing.
- Skin and eye contact must be prevented. Wear PPE appropriate to the hazard, as identified through the WPC Activity. Obtain a hazard assessment from an EHS Health and Safety Representative to determine the selection and use of PPE. PPE required for a wet-chemistry laboratory, which is often appropriate for handling ENMs, includes:
- Engineered Nanomaterial-Bearing Waste Streams
- Consider any material that has come into contact with UNPs (and that has not been decontaminated) as belonging to an ENM-bearing waste stream. This includes gloves, other PPE, wipes, blotters, and other disposable laboratory materials used during research activities.
- Do not put material from ENM-bearing waste streams into the regular trash or down the drain.
- Collect ENM-bearing waste in an appropriate sealing container such as a plastic bag. Until the container is sealed, keep it in the laboratory hood. The container must remain sealed unless adding waste to it. It should be managed as hazardous waste, including completing the Hazardous Waste label when accumulation begins, and placing it in an identified SAA. The identity of the waste must be given on the label. For example, “Wipes contaminated with trace levels of carbon nanotubes” provides an appropriate level of description. In addition to the applicable hazards on the label, add “NANO” or “<100 nm” to the “Other” hazardous properties section. When the bag is full, close it, take it out of the hood, and place it into a second plastic bag or other sealing container in an SAA.
- Characterize and manage ENM-bearing waste streams per the requirements of the Waste Management program (EH&S Manual Chapter 20). Be sure to consider the properties of all components, for example, solvents in which the ENMs may be dissolved or suspended.
- Emergency Procedures and Spills
- Refer to Work Process V, Emergency Procedures and Equipment, for Berkeley Lab policy and response procedures for chemical spills. Spills containing ENMs are generally handled in a manner similar to spills of other potentially hazardous materials, with the following additional requirements:
- Clean the spilled material using wet-wiping methods. Characterize, collect, and dispose of spill cleanup materials as ENM-bearing waste.
- Only HEPA vacuums that have been tested and certified by EHS may be used to vacuum nanomaterials. Do not dry-sweep or use compressed air.
- Consider using a walk-off mat such as a clean-room mat or “sticky mat” at access/egress points to reduce the likelihood of spreading nanoparticles. These are available through McMaster-Carr via the Laboratory’s Procurement & Property Web site.
- Refer to Work Process V, Emergency Procedures and Equipment, for Berkeley Lab policy and response procedures for chemical spills. Spills containing ENMs are generally handled in a manner similar to spills of other potentially hazardous materials, with the following additional requirements:
Work Process T. Specific Controls and Procedures — Chemicals with Explosive Properties
- General Information
- Berkeley Lab staff periodically use chemicals with explosive properties (i.e., explosives).
- For activities such as bioremediation or characterization studies, in which the focus is on the materials’ chemical and physical properties as opposed to their explosive properties, Berkeley Lab does not conduct activities in which an explosion or fragmentation hazard exists. Specifically, Berkeley Lab prohibits the synthesis, development, processing, blending, pressing, machining, testing, or detonation of explosives or assemblies containing explosives.
- An explosive, per the Department Of Energy Explosives Safety Standard DOE-STD-1212-2019, is “any chemical compound or mechanical mixture that is designed to function as an explosive, or chemical compound that functions through self-reaction as an explosive, and that, when subjected to heat, impact, friction, shock, or other suitable initiation stimulus, undergoes a very rapid chemical change with the evolution of large volumes of highly heated gases that exert pressures in the surrounding medium. The term applies to materials that either detonate or deflagrate.”
- Low explosives (see Figure 1) are materials that deflagrate: They burn more rapidly than materials undergoing normal combustion processes.
- High explosives detonate. Detonation is a process of combustion in which a shock wave is propagated at supersonic speeds. High explosives are divided into two classes: primary and secondary explosives.
- A primary explosive is extremely sensitive to impact, friction, heat, or electrostatic sources. Lead azide, lead styphnate, and mercury fulminate are examples of primary explosives. Primary explosives are often used in detonators or to trigger larger charges of less-sensitive secondary explosives. Primary explosives are prohibited at Berkeley Lab.
- Secondary explosives (also known as insensitive high explosives) are relatively insensitive to shock, friction, and heat. They may burn when exposed to heat or flame in small, unconfined quantities but normally require initiation from a primary explosive to detonate. Dynamite, trinitrotoluene (TNT), Cyclotrimethylenetrinitramine (RDX), Pentaerythritol tetranitrate (PETN), and 1,3,5,7-tetranitro-1,3,5,7-tetrazocane (HMX) are common secondary explosives. Solid (i.e. dry) secondary explosives in quantities exceeding 10 grams per control area are not permitted at Berkeley Lab.
- Materials that require review to determine the applicability of this policy include explosives defined by the Globally Harmonized System of Classification and Labeling of Chemicals and the United Nations Recommendations on the Transport of Dangerous Goods Volume I & II or 49 CFR Subpart C Transportation Regulations.
- Refer to Section 2: Hazards Identification of the Safety Data Sheet. The GHS Classification (Rev.10, 2023) Summary available through PubChem includes details of the GHS Explosives Hazard Class, Categories, Pictogram, and Hazard Statements.
- Refer to Section 14: Transportation of the Safety Data Sheet. The Security & Emergency Services Division GUIDANCE ON HAZARDOUS MATERIAL ASSESSMENTS provides detailed information on explosives defined by transportation or other regulations that require their review.
- The Procurement Restricted Items List, Restricted Chemicals and Gases list, CHEMR, includes specific materials requiring screening and approval prior to acquisition. See Work Process B. Chemical and Equipment Procurement for additional information.
- At Berkeley Lab, most explosives are used in dilute solutions. For explosives in solution concentrations ≤ 25% w/w, the main hazards are those associated with the solvent and the chemical as opposed to explosive properties of the material. However, if the solvent evaporates, or if the explosive crystallizes or precipitates, the primary hazard is associated with the explosive. See 45.7 Work Process P.1 Specific Controls and Procedures — Additional Time-Sensitive Chemicals, Section 9. Explosive When Dry, for applicable requirements.
- Control Measures
- The following activities involving explosives are prohibited at Berkeley Lab:
- Use of primary explosives
- Development, testing, processing, blending, pressing, and machining of explosives or assemblies containing explosives
- Operations that pose an explosion hazard, a metal fragment hazard, or a glass fragment hazard
- Activity leads are responsible for identifying explosives used in the work area. Review sources such as SDSs for specific compounds.
- Any quantity of explosives, no matter how small, is hazardous. However, the risk from amounts ≤10 milligrams of secondary explosives is significantly less than larger quantities. Therefore, quantities ≤10 mg of secondary explosives require no special precautions other than following the requirements in the CHSP and compliant storage.
- Divisions shall do a risk assessment in consultation with subject matter experts for use of explosives in quantities >10 mg of secondary explosives. Work involving these materials shall be added to a Work Planning and Control Activity. Consult the Work Planning and Control program (EH&S Manual Chapter 6).
- Airborne concentrations must be maintained at or below applicable occupational exposure limits.
- Visiting scientists periodically bring solid secondary explosives (typically in gram quantities) to Berkeley Lab to study their properties. These activities must be coordinated with the host division; however, the transport, handling, use, security, and custody of the material must be overseen by the visitor’s explosives safety experts and Berkeley Lab Security and Emergency Services Division (SES). A written security plan developed in collaboration with the visitors and SES must be approved by the hosting division and SES. Solid (i.e. dry) secondary explosives in quantities exceeding 10 grams are not permitted at Berkeley Lab.
- The written security plan shall address key elements including:
- How the material will be transported at LBNL sites, who is authorized to transport the material(s), and process details including necessary stakeholder notifications (e.g. SOC@lbl.gov).
- Security measures.
- Emergency response measures.
- The written security plan shall address key elements including:
- The following activities involving explosives are prohibited at Berkeley Lab:
- Training and Information
- Employees who either handle or who may be exposed to chemicals with explosive properties are required to complete Chemical Hygiene and Safety Training (EHS0348; or EHS0345 for Facilities personnel).
- Activity leads are responsible for on-the-job training specific to hazards and controls of these materials for their work activities. Information on hazards and minimum PPE requirements must be available to workers accessing work areas where these hazards are present such as through the entrance placard and co-located hazards in WPC activities. EHS Health and Safety Representatives are available to provide assistance.
- Consult Work Process Y, Container Labeling, for labeling requirements for primary and secondary containers.
- The entrance to the work area should be posted with a Caution Placard depicting hazards and emergency contact information.
- Substitution and Chemical Inventory Management
- Identify and use safer chemical alternatives (e.g., materials without explosive properties) if possible.
- If a safer chemical can’t be used, limit what you buy or borrow what you need from a colleague in your group or contact the Chemical Management System Coordinator (cms@lbl.gov) to assist you in finding a source of the chemical at Berkeley Lab.
- Keep working quantities at or below 10 mg if possible. Don’t stockpile chemicals.
- Conduct periodic cleanouts to prevent accumulating unneeded chemicals.
- Procure and use the minimum amount of material required for the operation, or
- Enter these materials into the Chemical Management System (CMS).
- Ventilation
Use a fume hood or other appropriate exhaust ventilation system when handling chemicals with explosive properties in a manner that may produce an airborne hazard (such as fumes, gases, vapors, and mists).
- Work Practices
- Control all ignition sources when handling explosives. This also applies to flammable and combustible solvents in which the material may be either dissolved or dispersed. Sources of ignition include open flames, smoking, hot surfaces, electrical and mechanical sparks, cutting and welding, static electricity, heat-producing chemical reactions, and anything that closes an electrical circuit (e.g., opening/closing a switch, plugging/unplugging a power cord, electrical motor or compressor switching on/off, etc.). Contact your EHS Health and Safety Representative or the Fire Marshal for assistance in identifying ignition sources. See Work Process N, Flammable and Combustible Liquids, for additional information.
- Storing and consumption of food is permitted in designated areas only. See Work Process J, Work Practice Controls, for additional information
- Open bottles or carboys slowly and carefully and wear protective equipment to guard hands, face, and body from splashes and vapors/gases.
- Wipe drips/residues from containers and work surfaces. Wash hands before leaving the work area and prior to consuming food/beverages.
- Personal Protective Equipment (PPE)
- Skin and eye contact must be prevented. Additional information may be found in Work Process T.7.c below.
- PPE shall be selected on the basis of the chemical hazard posed by the explosive compound and its solvent. PPE and shielding for protection against deflagration and detonation hazards should not be necessary because of the limits imposed on the types and quantities of explosives permitted and the stringent handling, storage and work practices required at Berkeley Lab.
- At a minimum, safety glasses with side shields, laboratory coats (coveralls are acceptable in shop settings), and closed-toe shoes will be worn when handling these materials. This is to be considered as minimum protection and must be upgraded if necessary.
- Additional PPE such as chemical goggles, face shields, chemical aprons, disposable coveralls, chemically resistant gloves, and respiratory protection must be worn if there is a greater chance of chemical exposure. An EHS Health and Safety Representative may be contacted for assistance in selecting appropriate gloves and respiratory protection. The use of respiratory protection requires an industrial-hygiene hazard evaluation and a medical clearance followed by a fit test and training by the Industrial Hygiene Group.
- Consult Work Process I.6, Eye and Face Protection, for guidance on the selection, uses, and limitations of safety glasses, chemical goggles, and face shields.
- Because many chemicals are skin-absorbers (i.e., agents that readily pass through the skin) it is important to select gloves that are chemically resistant to the material. Consult the PPE section. This contains a list of skin-absorbing agents and provides detailed guidance for selecting chemically resistant gloves.
- Gloves must be selected on the basis of their chemical resistance to the material(s) being handled, their suitability for the procedures being conducted, and their resistance to wear as well as temperature extremes. Improper selection may result in glove degradation, permeation of the chemical through the glove, and ultimately personal exposure to the chemical. This is a potentially serious situation. Consult Work Process I.5, Gloves, for guidance on the selection, uses, limitations, and disposal of chemically resistant gloves. An EHS Health and Safety Representative may also be contacted for assistance in selecting appropriate gloves.
- Storage
- Consult Work Process K, Chemical Storage for hazardous-material storage requirements, recommendations, and information on chemical incompatibility.
- Follow the storage guidelines in Work Process N, Specific Controls and Procedures — Flammables and Combustible Liquids if the material is either flammable or combustible.
- If several items of secondary explosives in quantities ≤10 milligrams are present in one area, separate the items by placing them in individual drip trays or flammable-storage cabinets to prevent inadvertent combinations exceeding 10 milligrams total mass. If the total inventory exceeds 10 milligrams in one area (i.e. per room), the requirements for chemicals with explosive properties per this work process must be followed.
- Any quantity of dry secondary explosives must be stored in compliant containers.
- Emergency Procedures
- Consult Work Process V, Emergency Procedures and Equipment, for emergency actions regarding chemical spill and personal exposure to chemicals.
- An emergency eyewash and safety shower should be located in all areas where flammable or combustible liquids are used. In the event of skin or eye contact, flush the affected area for at least 15 minutes and report to Health Services for evaluation and treatment. See Work Process V.7.a, Emergency Eyewashes and Safety Showers.
- Disposal
- Contact the Waste Management Group Generator Assistant for guidance or assistance on proper disposal of chemicals with explosive properties.
- Chemicals with explosive properties must be in a stable condition for transportation (e.g., adequately wetted) before placement in a SAA or WAA. Contact your Division’s Generator Assistant to ensure that your waste will be safe and compliant for pickup, storage, and transportation and meets off-site vendor’s disposal waste acceptance criteria.
- Chemicals with explosive properties which cannot be put into a stable condition cannot be safely disposed of as routine hazardous waste. These types of chemicals will require disposal through treatment under an Emergency Permit or other immediate external intervention. EH&S Division management approval is required.
- Source Requirements
- DOE Technical Standard DOE-STD-1212-2019, Explosives Safety.
- DOE Order (O) 470.3C, Chg. 21, Design Basis Threat (DBT).
- 24 CCR Part 9, California Fire Code.
Work Process U. Decommissioning or Transferring Equipment, Buildings, Laboratories, and Shop Spaces
- Line Managers, PIs, Chemical Owners, and Supervisor Responsibilities
- Line managers, PIs, chemical owners, and supervisors of laboratory and shop spaces, including equipment, are the most familiar with the hazards, historical spills, contamination, etc., associated with their spaces or equipment and are therefore responsible for ensuring that chemical, physical, biological, and radiological hazards, including respective hazard warning labels, have been removed prior to releasing these spaces, or their equipment, to Facilities Salvage or to new occupants.
- Contact an EHS Health and Safety Representative if assistance is needed with identifying hazards. In some cases, a separate hazard evaluation may be necessary.
- Before vacating an area or leaving the Lab, chemical owners must make sure that all hazardous materials are removed, transferred to new ownership, or properly disposed of. Work areas must be cleaned prior to transfer of ownership.
- The Division Director must assure all work is complete. Upon departure of a PI or shop supervisor, the Division Director will be accountable for any deficiencies not corrected by the PI/shop supervisor.
- Facilities Responsibilities. For Facilities construction, renovation, or building-demolition projects, the Facilities Project Manager must ensure that these hazards have been removed by line managers, PIs, and supervisors of laboratory and shop spaces prior to turning the building, space, or equipment over to the demolition/construction subcontractor or to Facilities Transportation. Building managers, division safety coordinators, and Facilities construction managers may be called upon to support this task.
- Areas or Equipment to Be Decontaminated. Line managers, PIs, and supervisors of laboratory and shop spaces are responsible for removing visible residues, hazard warning labels, standing liquids, and loose particulate material (whether a known or unknown material) on floors, bench tops, and shelves; and inside drawers, cabinets, refrigerators, surfaces of local exhaust enclosures (e.g., chemical fume hood and biological safety cabinet), and any other potentially contaminated surfaces. This also applies to any equipment that is to be moved, relocated, or sent to Salvage.
- Decontamination Processes
- It is recommended that surfaces be wiped down with mild detergents such as soap and water.
- A 10% bleach solution may be used for surfaces in labs where biological materials have been used.
- Use acid/alkaline neutralizers for acid or caustic spill areas.
- Attempting to clean up a mercury spill may spread the contamination throughout a location or building. Stay in one location and call the EHS Health and Safety Representative for assistance, and warn others to stay out of the spill area/room.
- Who Can Perform Decontamination
- Laboratory and shop employees who have taken CHSP training (EHS0348 or 345) can generally perform this work. In certain cases, the amount of work may require using the services of an outside contractor, including trained hazardous-materials specialists or abatement workers. Berkeley Lab and subcontractor employees with OSHA Hazardous Waste Operations and Emergency Response (HAZWOPER) training, under the direction of an EHS Health and Safety Representative, may also perform this work.
- Berkeley Lab custodians are not trained to perform this type of work; however, they may be contacted after the space has been evaluated and the room has been posted as being cleared by an EHS Health and Safety Representative (see section below). c. Contact an EHS Health and Safety Representative for assistance with this determination.
- Removal of Chemicals, Wastes, and Other Materials
- Equipment, supplies, products, and materials such as apparatuses, thermometers, gas cylinders, medical waste containers, sharps containers, sharps (needles and razor blades), trash, hazard warning labels, absorbent material, and other miscellaneous lab or shop material must be removed prior to vacating the space.
- For building demolitions, the project manager should be consulted to determine items that do not contain hazardous materials that are included in the demolition scope of work.
- In general, all chemicals and all chemical-related products must be removed. This includes cleaning compounds, surplus chemicals, stock solutions, experimental products/🚩samples🛑, and hazardous waste located in Satellite Accumulation Areas (SAAs). The current chemical owner must ensure the chemical inventory is accurate in the CMS system and all chemicals and 🚩archived🛑 samples are properly labeled. Current chemical owner may find new owners for unwanted chemicals first and transfer ownership of the chemicals by contacting the CMS Program Administrator. The new owner must accept the inventory. 🚩Research-produced samples that are retained must adhere to specific minimum labeling requirements for archived samples which include identifying an active LBNL employee point-of-contact familiar with the contents and hazard(s). 🛑Any leftover chemicals must be properly disposed of. A Waste Management Generator Assistant should be contacted for assistance.
- EHS Evaluation and Release of Laboratory and Shop Spaces, Including Equipment
- Following the decontamination of work or equipment surfaces, and the removal of chemical, physical, biological, and radiological hazards, EHS Health and Safety Representatives and radiological control technicians (RCTs) will perform a final inspection prior to the release of the space or equipment, depending on the radiological use in the space.
- EHS Health and Safety Representatives will evaluate for pH, evidence of debris, and “orphaned” chemicals, materials, 🚩or research-produced samples 🛑(as previously discussed), and will check for mercury in sink traps and floor surfaces.
- The radiological control technician will complete a green release tag indicating that the space or equipment has been cleared from radiological hazards (See the Radiation Safety program, EH&S Manual Chapter 21). An EHS Health and Safety Representative will release the space by posting the entrance (and individual pieces of equipment in some cases) with a release form that indicates the date and name of the Health and Safety Representative who evaluated the space.
- If individual pieces of equipment and supplies are to be moved to salvage or another building, and the owner of the equipment does not know whether hazardous materials are present, an EHS Health and Safety Representative may be contacted to evaluate these items and post them if they are safe to be handled by Berkeley Lab personnel or subcontractor employees.
- In cases where line managers, PIs or supervisors are familiar with identifying the hazards associated with their equipment and therefore do not need assistance from EHS, the Facilities Transportation Authorization Form (“TAF Form”) can be filled out without engaging EHS. This form is automatically sent to requesters through the Facilities Move Request process when move requests are initiated.
Work Process V. Emergency Procedures and Equipment
- General Spill Response Procedures
- In the event of a chemical spill, follow the S.W.I.M.S. procedure:
- STOP and think. Stop working. Stop the spill. Assess the situation:
- How big is the spill?
- Are there any injuries associated with the spill?
- Has it made contact with your skin or personal clothing?
- Can it be safely cleaned? NOTE: Follow the Spill Cleanup requirements listed below to make this determination.
- WARN others
- Call ext. 7911 or 9-911 if there is a medical emergency or danger to life, health or the environment.
- Alert people nearby.
- ISOLATE the area
- Restrict access to those involved in the spill cleanup.
- Determine the extent of the spill.
- Keep doors closed.
- MONITOR yourself carefully and completely
- Check yourself for any chemical contamination or signs/symptoms of exposure (e.g., wet clothing, skin or respiratory irritation).
- For medical emergencies, follow directions under the Emergency Preparedness: Emergency Guide.
- STAY in or near the area until help arrives
- Minimize your movements. Avoid spreading contamination to other areas.
- Have a person who is knowledgeable about the incident be available to talk to or assist emergency personnel.
- Notify your work lead.
- STOP and think. Stop working. Stop the spill. Assess the situation:
- In the event of a chemical spill, follow the S.W.I.M.S. procedure:
- Chemical Spill Cleanup Requirements
- You can clean up a chemical spill if ALL of the following requirements are met:
- You are NOT a high school student or a guest participating in an internship program.
- There is no potential for release to the environment. NOTE: Care must be taken to avoid spreading or tracking chemical contamination to other areas.
- There are no personal injuries resulting from the spill.
- You know what the chemical hazards are.
- The cleanup procedures are known and you have the proper spill-cleanup materials.
- You have the proper PPE to protect yourself during the cleanup.
- Two people can clean the spill up thoroughly within an hour.
- The spill does NOT involve elemental mercury. Special cleanup and monitoring procedures are required for mercury spills. Moreover, mercury contamination is easily tracked to other areas.
- If ALL of the above requirements are not met, or if you have any DOUBTS about your ability to safely and effectively clean up the spill, then:
- Leave the immediate area,
- Close the door,
- Stay close by and control access. Post the entrance with a warning such as “Spill — Do Not Enter” and
- Call (or have someone call) ext. 6999 for assistance.
- You can clean up a chemical spill if ALL of the following requirements are met:
- Other Chemical Spill Cleanup Considerations
- General Requirements
- Review these guidelines periodically — you must be familiar with them and know what to do before a spill occurs.
- Understand the hazards of the chemicals you use. Consult the Safety Data Sheets.
- Keep spill-cleanup kits in your work area.
- There are different types for acids, bases and solvents. These are commercially available through VWR Scientific. (The Laboratory has a contract with this vendor).
- It is important to note that absorbents and other materials used for spill cleanup need to be “inert” to the spilled material. For this reason, combustible materials such as sawdust and paper towels are generally inappropriate substitutes for the materials contained in spill kits.
- Wear the proper PPE to protect yourself. The minimum includes a lab coat (or coveralls), chemical goggles, and chemically resistant gloves rated for the chemical(s) of concern. Consult Work Process I, Personal Protective Equipment, for selecting and using eye/face protection and gloves.
- For mixed (i.e., radiological and chemical) spills, follow the S.W.I.M.S. guidelines: Stop your work and the spill; Warn others (contact the assigned radiological control technician or call the Urgent Radiation Safety Assistance at ext. 7277); Isolate the spill; Monitor yourself and the area (if appropriate); and Stay in the general area until help arrives.
- Ensure waste materials are properly contained and labeled and are placed in an approved SAA.
- Take Chemical Hygiene and Safety Training (EHS0348) for people who work in laboratories, or Chemical Hygiene for Facilities (EHS0345) for Facilities personnel.
- An emergency eyewash and safety shower must be located in all areas where acids and bases are used, and should be located in all areas where flammables, combustibles, laser dyes or solvents, and peroxide-forming compounds are used. In the event of skin or eye contact, flush the affected area for at least 15 minutes and report to Health Services for evaluation and treatment.
- Additional guidelines for the different types spill and unplanned events may be found in the Emergency Response Guide flip chart. A copy of the Emergency Response Guide flip chart must be posted in each Laboratory area.
- General Requirements
- Personal Injury from Exposure to Chemicals
- Berkeley Lab personnel are required to know the hazards and controls of chemicals in their work areas. This includes emergency first-aid procedures for inhalation, skin/eye contact, ingestion, and injection. Safety Data Sheets should be consulted for this information. Specific controls and procedures in Work Processes L through Work Process T provide additional measures for agents such as acids, bases, and reactive metals.
- In general, adhere to the following procedures for accidental exposures:
- Inhalation. If the material or its reaction/combustion products are inhaled, remove the person from the area and transport to Health Services (Building 26). For serious exposures, call 7-911. Lay the individual down and keep him or her warm and rested until medical care can be provided.
- Skin or eye contact. Flush the affected area for at least 15 minutes (See exceptions for phenol and hydrofluoric acid in Work Process L, Specific Controls and Procedures — Acids and Bases). Then immediately report to Health Services (Building 26, ext. 6266). Call 7-911 if the injury is serious or life-threatening.
- Ingestion. Call 7-911. If spontaneous vomiting appears imminent or occurs, help the person keep a clear airway. If victims are unconscious or cannot sit up, turn them on their side to help avoid possible aspiration of vomitus. Never give liquid to a person showing signs of sleepiness or who may become unconscious.
- Injection. This can occur from lacerations and punctures when handling sharps that are contaminated with chemicals. First stop the bleeding. Minor cuts and scrapes usually stop bleeding on their own. If they don’t, apply gentle pressure with a clean cloth or bandage. Rinse out the wound with clear water. Soap can irritate the wound. Report to Health Services (Building 26, ext. 6266). Call 7-911 if the injury is serious or life-threatening. c. It is important for personnel to identify the chemical involved in order to provide treatment. Provide the Material Safety Data Sheets or the chemical name to emergency response personnel and health care professionals.
- First Aid
- Dial 7-911 for assistance.
- People helping with the decontamination should wear lab coats, aprons, and chemical goggles. Glove selection is important:
- For phenol only, use Ansell Barrier, unsupported neoprene, supported polyvinyl alcohol (PVA), polyvinyl chloride (PVC), natural rubber, or ChemPro gloves.
- For phenol/chloroform mixtures, use either Barrier or supported polyvinyl alcohol (PVA) gloves.
- Remove contaminated clothing rapidly. Begin decontamination immediately, to minimize absorption.
- Irrigate or wipe exposed areas immediately and repeatedly with low-molecular-weight polyethylene glycol (PEG 300 or PEG 400), which can be diluted (with water) to 50% for easier application. Continue with treatment until the Fire Department arrives. Keep a supply of these reagents on hand for this purpose.
- Irrigation with water is not recommended, as water will merely dilute the phenol and expand the area of exposure.
- If a large area of the individual’s body is covered with phenol (too big to readily wipe with PEG), it is best to go under the emergency shower and await the arrival of the Fire Department. PEG can be applied to the most heavily contaminated section of skin.
- Flush exposed or irritated eyes with copious amounts of water or saline for at least 15 minutes.
- Emergency First Aid for Phenol Exposure
- Phenol and phenol/chloroform mixtures are common in Life Sciences research, and phenol is used in a number of Materials Sciences Division laboratories.
- Phenol is a combustible, highly corrosive chemical with a sweet, acrid odor.
- Phenol is well absorbed by all routes of exposure (inhalation, ingestion, skin and eye contact).
- Exposure by any route can cause systemic toxic effects as well as local corrosive effects.
- Eye contact will cause severe irritation, inflammation, and potentially irreversible loss of vision.
- Many phenol poisonings occur due to skin contact. While phenol is highly corrosive to tissue, pain perception may be depressed due to the chemical’s anesthetic properties, especially in concentrations of a few percent or less.
- Emergency Equipment
- Emergency Eyewashes and Safety Showers
- Plumbed emergency eyewashes and safety showers must be provided in areas where a splash hazard to corrosives, eye irritants, or chemicals that are toxic via skin and/or eye contact exists. They must be installed in the immediate work area at a location that can be reached by a blinded employee in an uncomplicated and unimpeded path within 10 seconds (approximately 50 feet). Specific locations shall be approved by EHS prior to installation. Exceptions to the criteria and procedures listed below may be granted by the EHS Deputy Director on a case-by-case basis.
- Plumbed emergency eyewashes and safety showers must be provided in areas where the following chemical types and quantities are either stored or used:
- Concentrated (greater than or equal to 1%) acids or bases (including acetic acid and ammonium hydroxide) – any amount
- Sensitizers – any amount
- Skin absorbers – any amount
- Formaldehyde solutions in concentrations greater than or equal to 0.1% – any amount
- Carcinogens or reproductive toxins in concentrations greater than or equal to 0.1% – any amount
- Dilute acids and/or bases (less than 1%) – 2 liters
- Eye irritants – any amount
- Organic solvents – 4 liters
- In addition, emergency eyewashes and safety showers are required in areas where an EHS Health and Safety Representative determines that emergency eyewash and safety shower are required because the operations, chemicals, or potential exposures pose a risk to eyes and/or body.
- At least one plumbed emergency eyewash and co-located safety shower should be installed in any new or remodeled location meeting any of the following conditions:
- Classified as “Hood” or “Hood and Gas” in the Berkeley Lab Space Database maintained by Facilities Department
- Having a general purpose or specialized chemical fume hood (including but not limited to lab bench, lab radioactive, walk-in, and acid digestion hoods)
- Used as an area to charge wet-acid storage batteries (e.g., for forklifts)
- When an eyewash is required, either a combination eyewash safety shower unit or an individual sink-mounted unit and overhead shower will be installed. Hand-held drench hoses are permitted as a supplement, but are not to be supplied in place of plumbed emergency eyewashes and safety showers. Self-contained, pressurized portable eyewash/safety shower units are permissible only for remote locations where the installation of a plumbed unit is not feasible. These shall be maintained in accordance with manufacturer’s requirements and are the responsibility of the owning division. The location of each emergency eyewash safety shower will be posted with a highly visible sign.
- The selection, installation, and use of emergency eyewashes and safety showers must comply with the most recent Berkeley Lab Facilities Installation Standard. Eyewashes and showers be vertically aligned to allow the eyes and body to be flushed simultaneously. Access to these facilities must remain open and free from obstructions at all times.
- Emergency eyewashes and safety showers must be inspected at least quarterly by Facilities personnel. Inspection tags must be filled out to document this activity. Notify the Work Request Center at ext. 6274 if a tag is found that shows a past due date.
- Fire Extinguishers

- Laboratories and shops using hazardous chemicals must have a fire extinguisher suitable for Class A (combustibles), B (flammable liquids), and C (electrical) hazards. For laboratories with combustible metals, an extinguisher suitable for Class D hazards is also required.
- Extinguishers must be located following NFPA 10 and should be placed along the exit path, preferably in corridors.
- The maximum travel distance for the ABC extinguishers varies from 30 to 50 feet, depending on the application. The maximum travel distance for Class D extinguishers is 75 feet.
- If additional extinguishers are needed for an area or if special extinguishers are needed for materials such as combustible metals, contact Berkeley Lab’s Fire Marshal for information concerning recommendations and requirements.
- Fire extinguishers are to be used only by Fire Department personnel or by employees in cases where the employee has already notified the fire department of the situation, is comfortable doing so, can do so safely, knows what is burning to select the appropriate extinguisher, and the attempt will not impede escape from the building if the fire is not extinguished. Fire Extinguisher Safety (web-based) (EHS0520) is required, as well as Fire Extinguisher Safety Retraining (EHS0531) annually thereafter. If desired, employees may also take Fire Extinguisher Safety (hands-on) (EHS0522).
- Do not attempt to fight a fire if you are not comfortable doing so, especially if it is large, fast-moving, or dangerous for other reasons such as chemical inhalation hazards. The safest course of action is always to leave the building and pull the fire alarm on your way out. Once at a safe location, call x7911 or 911 and inform the dispatcher of the fire location (building and room number) and what is burning, if known.
- Your safety should always be your primary concern. Before deciding to fight a fire, be certain that you:
- Alert people in the area and activate the nearest fire alarm
- Are comfortable using a fire extinguisher
- Are confident that you can fight the fire safely
- Know what is burning so that you can select the correct extinguisher
- Know the fire won’t block your exit if you can’t control it. A good way to ensure this is to keep the exit at your back.
- Are confident you won’t breathe any of the smoke or combustion products
- After use of a fire extinguisher, contact Berkeley Lab’s Fire Marshal for a replacement. Never return a used or damaged fire extinguisher to its wall-mounted bracket.
Work Process W. Training
- Activity Leads must ensure that employees complete training that is triggered by Work Planning and Control Activities.
- Training for employees working in areas where hazardous materials are present is completed at three levels:
- Berkeley Lab’s EH&S overview, Safety, Emergency Management & Trafficking Persons (LBL 0010). This course provides general hazard communication training.
- Chemical Hygiene and Safety Training (EHS0348) for people who work in laboratories, or Chemical Hygiene for Facilities (EHS0345) for Facilities personnel. These courses review the provisions of the CHSP and information on, chemical hazards and controls.
- Operation/procedure-specific training. This is to be provided individually or in small groups by the activity lead. The training reviews the hazards of an employee’s assigned work, the uses and limitations of controls, the warning signs of exposure to hazardous materials used in the operations (e.g., odors, irritation, etc.), and the emergency procedures for off-normal events. Line managers must ensure employees are trained in the hazards and controls associated with new materials introduced into the work area.
- EHS subject matter experts are available to assist line management in providing job-specific training.
- Collectively, this training addresses the following topics:
- The requirements of the Hazard Communication and Laboratory Standards
- Health and physical hazards
- Operations involving hazardous materials
- Applicable health standards (e.g., Cal/OSHA PELs and ACGIH TLVs)
- Use and location of SDSs
- Labeling requirements
- Purpose and use of control measures (work practices, substitution, engineering, administrative, and PPE)
- The warning properties of chemical releases (e.g., odors, eye irritation, etc.)
- The signs and symptoms of exposure
- Exposure monitoring and medical surveillance
- Spill response and emergency procedures
- Possible non-routine tasks
- Hazards of unlabeled pipes, wastes, etc.
- Newly identified physical or health hazards
Table W-1. Specific Hazard Training Classes
Work Process X. Safety Data Sheets
- General Requirements. Cal/OSHA updated its Hazard Communication Standard to be consistent with the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). It requires chemical manufacturers, distributors, and importers to communicate hazards through Safety Data Sheets (SDSs)- formerly Material Safety Data Sheets (MSDSs) – to downstream users.
- SDSs are required for all hazardous materials and must be readily accessible to LBNL personnel and visitors. This is done electronically as opposed to maintaining hard copies. Note: SDSs are not required for consumer products (e.g., Formula 409 all-purpose cleaner), provided they are used in the same manner as the general public.
- LBNL’s preferred source of SDSs is the ChemWatch (GoldFFX) Safety Data Sheets database, which is accessible from EH&S’s Safety Data Sheets and Chemical Information Resources. Additional SDS resources and information such as toxicological databases are also available from this site. LBNL personnel may also obtain SDSs from chemical manufacturers’ websites and other credible online resources.
- If an SDS for a chemical or product cannot be located, immediately contact the EHS Health and Safety Representative providing service to your Division to provide assistance. Within 7 working days, EHS will send a written request to the party responsible for the SDS (the manufacturer or importer of the chemical) for a complete SDS. You will be notified of the availability of the safety data sheet within 15 days of receipt from the manufacturer, producer, or seller.
- Personnel are requested to forward copies of SDSs that are obtained from manufacturers and other sources to chemsafety@lbl.gov for uploading to the online ChemWatch database.
- Whenever the employer (LBNL) receives a new or revised safety data sheet, such information shall be provided to employees on a timely basis not to exceed 30 days after receipt, if the new information indicates significantly increased risks to, or measures necessary to protect, employee health as compared to those stated on a safety data sheet previously provided.
- Safety Data Sheets – Required Information
- The information contained in the SDS is mostly the same as the MSDS, except now SDSs are required to be presented in a consistent, 16-section format. (Refer to the Federal OSHA Hazard Communication Website for additional information.)
- Identification: product identifier; manufacturer or distributor name, address, phone number; emergency phone number; recommended use; restrictions on use.
- Hazard(s): all hazards regarding the chemical including hazard class and category and label elements – signal word, hazard statement, pictograms and precautionary statements.
- Composition/information on ingredients: chemical name, synonyms, and CAS number.
- First-aid measures: includes important symptoms/effects, acute, delayed; required treatment.
- Fire-fighting measures: suitable extinguishing techniques, equipment, chemical hazards from fire.
- Accidental release measures: lists emergency procedures, protective equipment, proper methods of containment and cleanup.
- Handling and storage: precautions for safe handling and storage, including incompatibilities.
- Exposure controls/personal protection: occupational exposure limits and appropriate engineering controls and PPE.
- Physical and chemical properties: such as odor, odor threshold, flash point, boiling point, explosive limits, evaporation rate, vapor pressure and vapor density.
- Stability and reactivity: chemical stability reactivity hazards.
- Toxicological information: routes of exposure, related symptoms, acute and chronic effects, numerical measures of toxicity.
- Ecological information: environmental impact resulting from releases.
- Disposal considerations: proper disposal practices.
- Transport information: transportation classification for shipment by road, rail, sea or air.
- Regulatory information: relevant safety, health and environmental regulations.
- Other information: includes the date of preparation or last revision.
- If no relevant information is available for any given section, it shall be marked to indicate so.
- Trade Secrets. The specific chemical identity, including the chemical name and other specific identification of a hazardous chemical, may be withheld from the SDS, provided that:
- The identity is a trade secret. A “trade secret” is any confidential formula, pattern, process, device, information, or compilation of information that is used in an employer’s business, and that gives the employer an opportunity to obtain an advantage over competitors who do not know or use it.
- The claim that the information withheld is a trade secret can be supported.
- Information contained in the SDS concerning the properties and effects of the hazardous chemical is disclosed.
- The SDS indicates that the specific chemical identity is being withheld as a trade secret.
- The specific chemical identity is made available to health professionals, employees, and designated representatives upon request in accordance with applicable provisions outlined in the Cal/OSHA Hazard Communication Standard
- The information contained in the SDS is mostly the same as the MSDS, except now SDSs are required to be presented in a consistent, 16-section format. (Refer to the Federal OSHA Hazard Communication Website for additional information.)
- SDS Requirements for Chemicals Produced in LBNL Laboratories
- LBNL is regarded as a chemical manufacturer or distributor by Cal/OSHA if chemicals produced in laboratories are shipped off site. An SDS containing the elements outlined in Section 2, must be prepared and shipped with the chemical.
- If the chemical is produced for use at LBNL or its satellite locations, an SDS is not required.
- Refer to Work Process Z for additional details and hazard communication requirements for chemicals produced in laboratories.
Work Process Y. Container Labeling
- General Requirements
- Labels are required for all primary and secondary containers of chemicals. 🚩Research produced samples require labeling consistent with secondary containers when space permits; options for practical size limitations outlined below.🛑 Primary containers are the original containers received from the manufacturer, distributor or vendor. Secondary containers are jars, cans, squeeze bottles, and other containers to which chemicals are transferred from a primary container by an individual.
- Labels must be clearly legible, prominently displayed and written in English.
- LBNL personnel shall not remove or deface labels on primary containers unless they are emptied and re-purposed for a different use such as accumulating waste.
- The labeling provisions of this Work Process do not apply to Waste Containers. Consult Guidelines for Generators to Meet HWHF Acceptance Requirements for Hazardous, Radioactive, and Mixed Wastes at Berkeley Lab (PUB-3092) for labeling waste containers.
- Primary Container Labeling
- Cal/OSHA updated its label format to be consistent with the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). Labels on primary containers shipped by manufacturers must have the following elements:
- Product identifier or chemical name;
- Signal word;
- Hazard statement(s);
- Pictogram(s);
- Precautionary statement(s); and,
- Name, address, and telephone number of the party who produced the chemical.
- Refer to the Cal/OSHA Publication, or Federal Hazard Communication Website, for a detailed explanation of each of the required label elements.
- Cal/OSHA updated its label format to be consistent with the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). Labels on primary containers shipped by manufacturers must have the following elements:
Note: Older containers at LBNL do not have to be relabeled to meet the new format.
- The following is an example of a label with the new format:
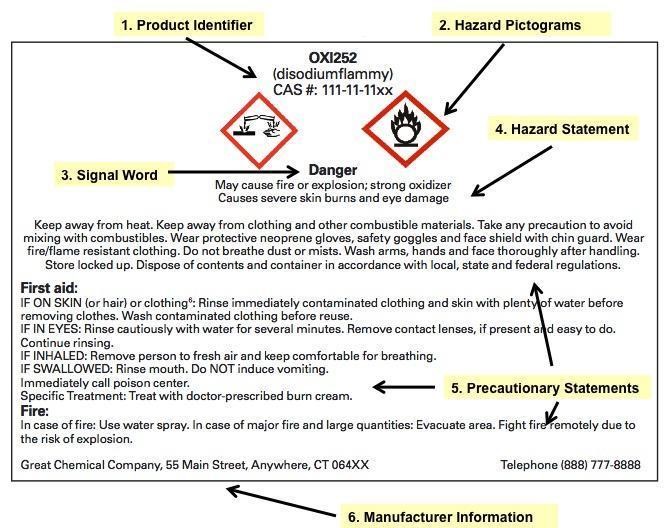
- Secondary Container Labeling
- Secondary containers are jars, cans, squeeze bottles, and other containers to which hazardous materials are transferred from a primary container by an individual.
- All secondary containers, containing hazardous or non-hazardous materials must be labeled with the content identification at the minimum. Name of the person responsible and date of transfer are recommended to be labeled on secondary containers.
- Secondary containers of hazardous materials must be labeled with content identification and words, pictures, symbols, or combination thereof, which provide at least general information regarding the hazards of the chemicals as a whole, with the following exceptions:
- Containers used in ongoing experiments in research labs: These may include research samples, and containers, vessels and flasks, which are connected to equipment or an apparatus,
- Containers for immediate use in shops and other technical areas: These are containers that are under the control of the user and will be used in the same work shift.
For example, a squeeze bottle containing acetone should be labeled as follows: “Acetone – Flammable, Irritant.” This information may be added to the secondary container by a number of different ways such as using a label maker, writing on the container with a pen, or by using a commercially available label. Pre-labeled squeeze bottles are also acceptable. Contact EHS for available labels.
- The information on the primary container’s label (i.e., signal word, pictogram, hazard statement, precautionary statement and contact information) is not required to be transcribed onto secondary containers.
- Product names such as X-14 are permissible provided that they match the name on the label of the primary container. Listing individual chemical components of mixtures is not required.
- Labeling 🚩Actively Used🛑Small Containers (Vials and Tubes) in Laboratories. There may be practical limitations to carrying out the above labeling requirements on small containers such as sample vials and tubes. Alternatives such as labeling a tray or rack that holds the containers or applying a numbering or coding system are permissible provided that the material’s identity and hazards are readily accessible (e.g., by means of a lab notebook, a spreadsheet, or some equivalent means) to research personnel in the laboratory and that they understand the system. 🚩Note: sample information written on a label/sticker placed onto the vial or test tube is recommended to prevent the information from being lost over time due to handling.🛑
🚩Sample storage locations (cabinet, drawer or tray) must be labeled with the appropriate hazard communication and identified as sample storage locations.🛑 - 🚩🚩Labeling for Research-Produced Chemicals/Mixtures/Samples Retained After A Responsible Individual Leaves the Lab (Archived Samples). Samples that are to be retained for storage after the responsible individual (e.g. the research generator) leaves the lab must labeled prior to their departure from the Lab with the hazard(s) and an active LBNL employee point of contact (e.g. the Principle Investigator) who has agreed to accept responsibility for proper management, including hazardous waste requisition. Alternatives provided above for actively used small containers are acceptable. EHS provides labels. Field support may be requested by emailing labsafety@lbl.gov. Archived samples require the same chemical storage and segregation that is required of active samples. Refer to Work Process K for additional information on chemical storage.🛑
- Prepackaged Kits Containing Individual Containers. Kits containing individual containers of hazardous materials are often used in both laboratory and non-laboratory work environments. The outer packaging of a kit is considered to be a primary container. Therefore individual containers require no additional labeling if they get separated from the kit.
- Process Container Labeling. Process containers such as baths and tanks may be identified with signs, placards, or operating procedures in lieu of affixing labels to individual containers.
- Labeling Containers for Chemicals Produced in LBNL Laboratories
- If chemicals are produced in LBNL laboratories, and are either shipped offsite or used on site at LBNL or its satellite locations, the hazard communication requirements (including labeling requirement) are described in Work Process Z.
- Research samples in general are treated as described in section 4 and 5 of this work process.
- Exception: If research samples will be transported offsite or used by another person, the owner of the research sample must follow the requirements described in Work Process Z section 4 to ensure the hazard information of the sample is communicated to the user.
Work Process Z. Hazard Communication Requirements for Chemicals Produced in LBNL Laboratories
- General Requirements. Chemicals produced in laboratories must, at a minimum, be evaluated for their hazards. Other requirements such as training, and the production of SDSs and labels vary depending on whether the materials will be shipped off site or used on site. Details are provided in the following section. Table Z-1 is a summary of these requirements.
- Chemicals Produced in LBNL Laboratories and Shipped Off Site
- LBNL is regarded as a chemical manufacturer or distributor by Cal/OSHA if chemicals produced in laboratories are shipped off site. Therefore, LBNL is required to comply with the requirements to identify chemical hazards of the materials produced and to communicate them through SDSs and container labels that are shipped with the chemical. There is no exemption based on quantity. Select toxins, regulated by the Centers for Disease Control and biological materials are exempt from these requirements.
- Hazard classification, and the creation of SDSs and labels are greatly simplified for chemicals which have already been evaluated by another party. For example, if benzene is produced in an LBNL laboratory, its SDS and label can be downloaded and used from LBNL’s SDS provider, ChemWatch®.
- For new or unique chemicals, hazard classification and the creation of new SDSs and labels are required and must be done in accordance with Cal/OSHA’s mandated prescriptive methodology. The process for classifying chemical hazards is described in Work Process E, Work Process Y and Work Process X list the required content and elements for labels and SDSs, respectively.
- Due to the complex and prescriptive requirements for classifying new chemicals, and for developing SDSs and labels, researchers may consult with the CHSP manager for assistance.
- For SDSs and labels that are shipped with chemicals, line managers shall ensure that:
- The recipient is contacted (e.g., via e-mail) prior to shipping.
- Shipping is coordinated with Facilities Material Services (ext. 5084). Only qualified individuals in Facilities Material Services may pack and ship these materials off site.
- SDSs and labels are updated with any new information regarding the hazards of a chemical or ways to protect against the hazards.
- Chemicals Produced in Laboratories and 🚩Actively🛑Used On Site. The following requirements apply to chemicals that are produced in LBNL laboratories but will be used on site use, as opposed to shipping them to an off site location. This applies to chemicals that are saved for future 🚩on site🛑use or that are shared with others on site. “On site” includes the main LBNL campus and its satellite locations.
- The chemical must be evaluated to determine if it is hazardous using the protocol outlined in Section 2, above.
- SDSs are not required.
- Labels are required. Minimum information includes product information such as chemical name or structure and at least general information regarding the hazards of the chemicals. Note: the Cal/OSHA primary container label elements, including signal word, pictogram, hazard statement, precautionary statement and contact information are not required.
- If the composition is unknown, label the container with some other means of identification such as researcher name, date, and reference to a lab notebook page.
- 🚩There may be practical limitations for labeling small vials or multiple containers kept in a rack, tray or cassette. Refer to Work Process Y for additional information and guidance on labeling.🛑
- There may be practical limitations for labeling small vials or multiple containers kept in a rack, tray or cassette. Refer to Work Process Y for additional information and guidance on labeling.
- Training is required. At a minimum, complete Chemical Hygiene and Safety Training (EHS0348) and Safe Handling of Engineered Nanoscale Particulate Matter (EHS 0344), if the chemical is an engineered nanomaterial.
- Address the hazards and controls of the chemical in the WPC Activity for which it is used.
- Hazard Communication Requirements for Transporting Research Samples. Employees who transport research samples either by hand or in a passenger vehicle for use by another person must follow the hazard communication requirements described above and the packaging requirements in Work Process C, Transporting Hazardous Materials.
Table Z-1 Hazard Communication Requirements for Chemical Substances Produced in Laboratories
| Usage Condition | Classify Hazards | Produce SDS | Label Container | Train End User | WPC |
| Shipped off-site | Yes1 | Yes2 | Yes3 | No | NA |
| For use on site 4 | Yes1 | No | Yes5 | Yes6 | Yes7 |
| Transportation of chemical (User is an LBNL person)8 | Yes1 | No | Yes9 | Yes6 | NA |
| Transportation of chemical (User is a non-LBNL person)10 | Yes1 | Yes2 | Yes3,9 | No | NA |
- Determine the chemical hazards in accordance with Cal/OSHA’s hazard classification and categorization process outlined in Work Process E.
- Produce an SDS for the chemical. See Section 2.
- Label in accordance with the Cal/OSHA HCS. See Section 3.
- “On site” includes the main LBNL campus and its satellite locations.
- Label with the chemical name and principal hazard. See Section 3.
- Training courses EHS 0348 and 0344 (for engineered nanomaterials) are required, plus any information on the hazards and controls associated with the substance produced in the laboratory.
- Address the hazards and controls of the chemical in a WPC Activity.
- Transportation by hand or in a passenger vehicle for use by either the sender or other LBNL personnel. See Work Process C.
- In addition to chemical name and hazard, list the name and phone number of the sender and recipient. See Work Process C for additional information and requirements.
- If a substance will be used by non Berkeley Lab personnel, the hazard classification, labeling and SDS requirements of the Cal/OSHA Hazard Communication Standard apply.
- 🚩🚩Chemicals Produced in Laboratories Not in Active Use (Archived Samples). Refer to Work Process Y for additional information and guidance on labeling. 🛑
Work Process AA. Posting Area Entrances

- Area safety leaders must ensure that entrances to technical areas are posted with a Caution Placard that indicates the hazard types in the work area (such as corrosives and carcinogens) as depicted by hazard icons, minimum PPE requirements, and emergency contact information.
- Caution Placards come in two template sizes:
- Follow the instructions and use the hazard icons to create the placards.
- Note: Use the icons provided in the PowerPoint file.
- If there is a hazard in your work area for which there is no available icon, or if you have an icon you wish to add to the list, consult with the CHSP manager. Do not create and use your own icons.
- If interim conditions or activities in a technical area pose temporary hazards that require additional controls, supplemental signs may be posted. Use the Posting Template. The description of hazards, controls, and points of contact must be agreed upon by an EHS subject matter contact and the division representative (such as the area safety leader, work lead, or principal investigator). Post the sign in a prominent location.
- The Emergency Response Guide flip chart must be posted in laboratories and work areas where chemicals are used. These flip charts instruct personnel on the actions to take during off-normal events (chemical, radiological and biological spills, fires, and earthquakes). Please contact the Security and Emergency Services Division to obtain a flip chart for your area.
Work Process BB. Designated Areas
- Designated areas are specific locations within a laboratory for work involving particularly hazardous substances. Their purpose is to ensure that proper controls are in place and that all activities involving these higher-hazard materials are confined within the designated area.
- Designated areas can be a piece of equipment, such as a fume hood or a centrifuge, or they can be entire laboratories. However, it is best to limit the number and size of designated areas to the minimum needed because there are additional control procedures required. Refer to Work Process M for more information.
- The activity lead must establish and post designated areas.
- If an entire lab has been established as a designated area, then the “Caution” Entrance Placard with the applicable icons serves as the designated area posting. See Work Process AA. Additional designated area postings are not required.
- If only specific areas within a lab, such as a piece of equipment, fume hood, lab bench, etc. are established as designated areas (without the entire lab being so designated), then the Designated Area sign must be used for posting these specific designated areas.
- Employees working in designated areas must be informed of the hazards and controls of the materials used.
Work Process CC. Exposure Assessments, Monitoring, and Medical Consultation
Hazard assessments are conducted to identify hazards of chemicals that are used in work activities for establishing engineering, work practice, administrative and training controls. These are conducted by Activity Leads and safety professionals for developing WPC Activities and for providing consultation to the Laboratory community. Hazard assessments may precede exposure assessments, which are conducted by EHS professionals to ensure that protective measures are implemented and to ensure worker health compliance with applicable regulations or other requirements. Exposure assessments are required prior to issuing and using respiratory protection equipment.
- Exposure Assessments
- An exposure assessment is a more formal evaluation process performed and documented by EHS professionals to determine the risk of personnel exposure to hazardous chemical or physical agents, and the adequacy of hazard controls. Results of exposure assessments are used to validate or improve hazard controls, to extend the same controls to employees with similar exposures, to provide employees with appropriate medical tests and examinations (i.e., medical surveillance) to monitor employee health, and to demonstrate compliance with regulations. The Exposure Assessment Program program (EH&S Manual, Chapter 4) describes this process in more detail.
- Exposure assessments may be either qualitative or quantitative assessments of risk. Qualitative exposure assessments result from observation and the use of professional judgment, whereas quantitative assessments involve conducting measurements (i.e., exposure monitoring) or by estimating or modeling of exposures.
- Exposure Monitoring
- The main type of exposure monitoring is personal air sampling. There are other techniques that can assist in determining employee chemical risk, including area air sampling, dermal exposure assessment, wipe sampling, and bulk sampling (see Definitions). Not all chemical contaminants have established analytical methods; however, those that do may be monitored by:
- Personal air sampling. Air is sampled from a worker’s breathing zone and analyzed to determine the presence and concentration of airborne contaminants.
- Area air sampling. Air concentrations of an agent are collected in a specified area to determine whether the sample meets an established criterion. This method is most often used after a cleanup or remediation operation, such as lead or asbestos.
- Dermal monitoring. A technique used to assess a worker’s skin exposure to a given chemical. This strategy is used for substances that have properties that may allow them to cross the intact skin into a worker’s system.
- Wipe sampling. Surfaces (such as bench tops) are tested to determine the presence and amounts of residual contaminants on surfaces.
- Bulk sampling. Materials are collected and analyzed to determine the presence and amounts of contaminants such as lead and asbestos. These are normally collected before demolition, construction, and renovation activities.
- The main type of exposure monitoring is personal air sampling. There are other techniques that can assist in determining employee chemical risk, including area air sampling, dermal exposure assessment, wipe sampling, and bulk sampling (see Definitions). Not all chemical contaminants have established analytical methods; however, those that do may be monitored by:
- Employee exposure to airborne chemicals will be kept at or below the occupational exposure limits referenced below in Section 2.d of this work process. The potential for exposure to airborne chemicals is lower in laboratories than in industrial settings. This is because smaller quantities of chemicals are used, and they are normally handled in fume hoods or other systems, such as glove boxes, and workers have usually received advanced training.
- However, if there is reason to believe that use of a chemical may produce airborne levels above applicable limits (regardless of occupational setting), air exposure monitoring will be conducted.
- If initial monitoring indicates exposures higher than half of the applicable limits, follow-up monitoring will be conducted. Moreover, controls (such as work practices, training, personal protective equipment, engineering, ongoing air monitoring, and medical surveillance) will either be put into place or enhanced, based on the judgment of the industrial hygienist and any specific Cal/OSHA standard that may apply.
- Monitoring will be terminated when successive follow-up measurements indicate exposures are below half of the applicable occupational exposure limit (OEL).
- Exposure assessments are conducted by EHS Health and Safety Representatives and other EHS professionals to identify the potential for employee exposure to hazardous materials and to ensure proper control measures are in place.
- Exposure assessments may be conducted for operations involving the use of particularly hazardous substances, unstable/reactive compounds, chemicals regulated by Cal/OSHA substance specific standards, and for other chemicals and operations as deemed appropriate by an EHS Health and Safety Representatives
- Exposure assessments may also be done in response to a Health Services referral, or when a concern is expressed by an employee, a supervisor, or a work lead.
- Occupational Exposure Limits (OELs) and Interpretation of Monitoring Results
- Air-sampling results are compared with exposure limits to determine whether the potential for hazardous exposure exists. The following occupational exposure limits are used:
- The Cal/OSHA 8-hour time-weighted average permissible exposure limit (PEL) for a substance: This is a legal exposure limit that must not be exceeded.
- The American Conference of Governmental Industrial Hygienists Threshold Limit Values (ACGIH TLVs): These are intended as recommended 8-hour, time-weighted average concentrations, though they are included in 10 CFR 851, the DOE Worker Safety and Health rule.
- The Cal/OSHA action level (AL): An AL, which is generally one-half the substance’s PEL, normally triggers additional monitoring and/or medical surveillance requirements.
- The Cal/OSHA and ACGIH short-term exposure limit (STEL) is the average concentration to which workers can be exposed for a short period of time (15 minutes) without suffering from irritation, chronic, or irreversible tissue damage or narcosis, provided that the daily 8-hour time-weighted average exposure limit is not exceeded. The STEL is intended to protect workers from acute toxic effects.
- Cal/OSHA and ACGIH ceiling limit (C): The concentration that may not be exceeded during any part of the working exposure (workday).
- Several individual substances have both Cal/OSHA and ACGIH exposure limits. In some cases, the values of these two limits are different. LBNL uses the lower of the two limits to interpret exposure results.
- The Cal/OSHA exposure limits (8 CCR 5155, Airborne Contaminants) are available online.
- Various criteria exist for evaluating the results from wipe and bulk samples. Consult the EHS Health and Safety Representative providing support to your Division for more information.
- Air-sampling results are compared with exposure limits to determine whether the potential for hazardous exposure exists. The following occupational exposure limits are used:
- Employee Notification of Monitoring and Recordkeeping
- The EHS Health and Safety Representative conducting the exposure-monitoring must give written notification of the monitoring results to the employee (and employee’s supervisor) in accordance with the specific Cal/OSHA requirements for that substance. Where no criterion exists, monitoring results will be provided within 15 days of receiving analytical results from the laboratory performing the analyses.
- Health Services must also be notified of exposure monitoring results.
- Monitoring records will be managed by the EHS Research Support Team.
- Consultations
- Medical consultations and examinations related to employee exposure are provided by Health Services (ext. 6266) to any employee exposed at or above an action level (or in the absence of an established AL, one-half the lower of the Cal/OSHA PEL or the ACGIH TLV); when an employee develops a sign or symptom of exposure to a hazardous material; or when an uncontrolled event such as a spill, leak, or explosion takes place in which there is a likelihood of employee exposure.
- Anyone with a concern or question may request a medical consultation. Health Services should also be consulted by women who are either pregnant or intend to become pregnant.
- Medical consultations and examinations will be conducted in accordance with Berkeley Lab’s Health Service Program policies and procedures, which are described in the Health Services program (ES&H Manual, Chapter 3).

