
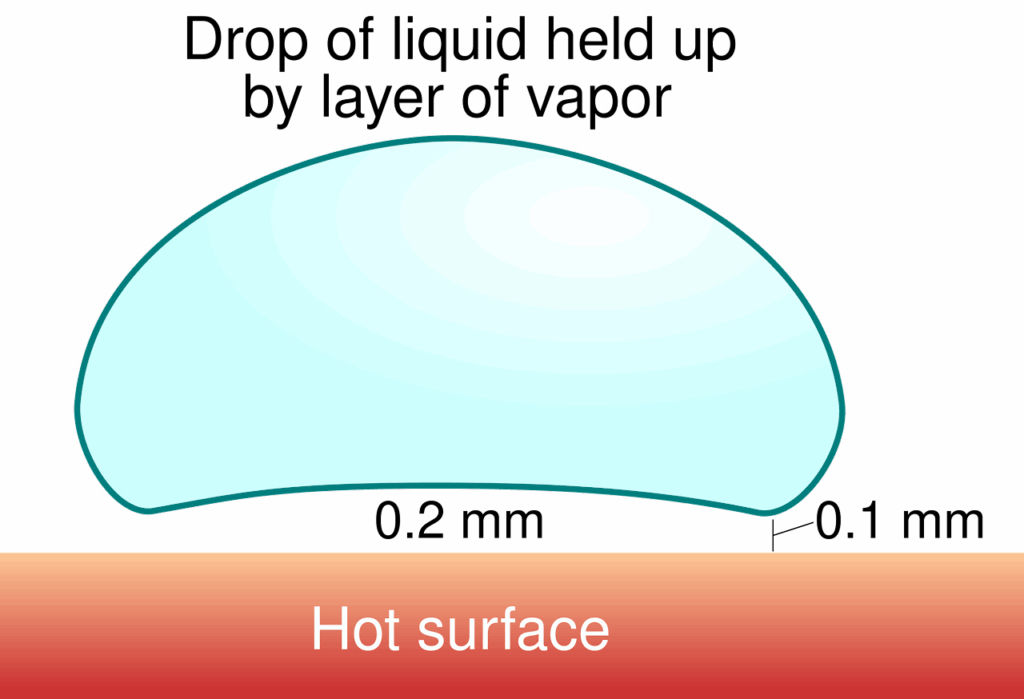
The Leidenfrost effect is well explained by the Wikipedia article, found here. In brief, it is a phenomenon where a liquid that contacts a surface at a temperature far enough above its boiling point will hover on a layer of expanding vapor. The boil-off vapor then acts as an insulating layer between the liquid and the surface, slowing heat transfer. As a secondary consequence, the liquid skates easily across the surface on a low-friction layer of gas, much like a puck across an air hockey table. This phenomenon is observed in cryogenic liquids on contact with room-temperature objects, and is also commonly seen when water droplets contact a very hot cooking surface – in fact, that’s how I was taught to test if the skillet was ready for pancakes.
The Leidenfrost effect works to our advantage when handling cryogenic liquids. A small splash of cryogenic liquid over the skin tends to skate off on a cushion of expanding gas, with a heat transfer rate so low that it feels like nothing more than reaching into the freezer.
But this can also lead to a false sense of security. The protectiveness of the Leidenfrost effect relies on perfect conditions for the liquid to skate freely off the body. Any place where the liquid might collect, such as local depressions (a cupped hand, a shirt pocket, a rolled cuff), prevents the liquid from running off and can lead to severe burns. Additionally, some clothing materials may absorb cryogenic liquids, holding them against the body. See the PPE Selection Guide for more information on how to avoid this.
It is also important to note that there is no such protection when handling solid objects that have been cooled to cryogenic liquid temperatures. Metal parts that are in contact with a cryogenic liquid, such as piping, valves, and fittings, can and will cause severe burns on contact with skin.
Absorbent Materials and Worn Items Can Be Dangerous
The Leidenfrost effect only works on non-porous, non-absorbent materials. Recently, we learned the hard way that absorbent materials can and do absorb liquid nitrogen and can cause serious burns. Even a small, incidental splash of liquid nitrogen can be absorbed by some materials, especially absorbent cloth, trapping the ultra-cold liquid against the skin. Therefore, it is important to ensure that you have no absorbent materials near your hands or wrists while performing work with cryogenic liquids. Similarly, avoid thermally conductive materials such as metal watches and jewelry that may become ultra-cooled by a liquid nitrogen splash and cause burns where the thermally conductive material touches the skin.
Some Notes on Cryo Gloves
The outer material of cryo gloves is a tightly woven fabric to prevent cryogenic liquids from absorbing into the glove material. It’s important to never attempt to use a glove made of porous or loosely woven fabric to handle cryogenic liquids.
There are also two main styles of cryo gloves: One with wide openings at the wrists, and one with elastic cuffs at the wrists. Berkeley Lab recommends the gloves with loose, wide openings at the wrists, and that cryo gloves be over-sized for the hands of the person wearing them. The reason for this is to protect against the possibility of trapping cryogenic liquid within the glove against the hand. If cryogenic liquid enters the glove, the glove will need to be removed quickly to minimize the potential of a cryo burn. Elastic cuffs make it harder to remove the glove, while gloves with a wide, loose opening at the wrist can be thrown off quickly with a flick of the arm.

Cryo gloves with wide, loose openings at the wrist
Recommended


